Ethyl nitrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
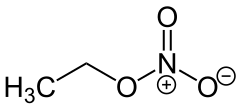
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethyl nitrate | |||||||||||||||
| other names |
Ethyl nitric acid |
|||||||||||||||
| Molecular formula | C 2 H 5 NO 3 | |||||||||||||||
| Brief description |
colorless, pleasant smelling liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 91.07 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.11 g cm −3 |
|||||||||||||||
| Melting point |
−112 ° C |
|||||||||||||||
| boiling point |
88 ° C |
|||||||||||||||
| Vapor pressure |
66.5 h Pa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| Refractive index |
1.3852 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−190.4 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethyl nitrate , the nitric acid ester of ethanol , is a transparent, highly explosive liquid with a pleasant odor.
Natural occurrence
According to studies by the University of East Anglia , ethyl nitrate is also biogenic in addition to its artificial production . It is highly likely that the origin is aquatic bacteria.
presentation
Ethyl nitrate is formed when concentrated, nitric oxide-free nitric acid acts on ethanol. The esterification reaction is exothermic with a molar heat of reaction of −25.9 kJ mol −1 . In order to isolate the resulting ethyl nitrate, it must be carefully distilled off. The production of ethyl nitrate is remarkably simple and inexpensive, but at the same time very dangerous, since the ethyl nitrate has to be converted into the gas state in which it is highly explosive for purification.
In order to prevent the ethanol from being oxidized by a side reaction , even the smallest traces of nitrous acid must be removed by adding urea nitrate . Otherwise an autocatalytic reaction can occur, which can be explosive.
properties
Ethyl nitrate has a boiling point of 88 ° C under normal pressure and is heavier than water. According to Antoine, the vapor pressure function results from log 10 ( P ) = A - ( B / ( T + C )) ( P in Torr, T in ° C) with A = 7.145, B = 1329 and C = 224.0 in the temperature range from 0 ° C to 88 ° C. The molar enthalpy of vaporisation is the boiling point of 33.9 kJ · mol -1 , at 25 ° C 36.4 kJ · mol -1 . An azeotrope boiling at 74.35 ° C is formed with water . The azeotrope is 22 Ma% water and 78% ethyl nitrate Ma. The compound forms highly flammable vapor-air mixtures. It has a flash point of 10 ° C. The lower explosion limit (LEL) is 3.8% by volume (140 g / m 3 ). An upper explosion limit (UEL) cannot be determined due to the self-destruction of the compound. The vapors of ethyl nitrate, like those of the chemically closely related methyl nitrate , are explosive even in the absence of air, which, in connection with the high volatility of the substances, harbors high potential hazards. It is just as sensitive to impact as nitroglycerin , but is inferior to this in terms of explosive power. Since there is not enough in the molecule oxygen atoms are, at the existing carbon - and hydrogen -atoms completely oxidize, it has a negative oxygen balance . Therefore, the plumes created during detonation contain flammable gases such as hydrogen and carbon monoxide. Oxygen must be added for complete combustion:
- .
The heat of combustion is −1355.8 kJ mol −1 .
Explosion parameters
The connection is particularly explosive due to shock, impact, friction, fire and other sources of ignition and, when handled, falls under the Explosives Act . Important explosion parameters are:
Table with important explosion-relevant properties: Oxygen balance −61.5% Nitrogen content 15.24% Normal gas volume 1228 l kg −1 Explosion heat 4133 kJ kg −1 (H 2 O (l))
3739 kJ kg −1 (H 2 O (g))Specific energy 1108 kJ kg −1 (113.0 mt kg −1 ) Lead block bulge 42.0 cm 3 g −1 Detonation velocity 5800 m s −1
use
Ethyl nitrate has little commercial use, for example to increase the cetane number in diesel fuels (similar to other alkyl nitrates used for this purpose ). In its pure form, it is weaker and less safe to handle than nitroglycerin. However, due to the easy availability of the starting materials, it is often produced illegally and used by terrorists. If it is used as an explosive, it is often mixed with oxidizing substances such as ammonium nitrate in order to balance the oxygen balance and to optimally use the explosive power of the substance.
See also
Individual evidence
- ↑ a b c d e f g Entry for CAS no. 625-58-1 in the GESTIS substance database of the IFA , accessed on December 8, 2012(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-250.
- ↑ Entry on ethyl nitrate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers and / or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ Antje Findeklee: Alkyl nitrates of natural origin . Spektrum.de, 23 August 2002.
- ↑ a b c d e P. Gray, MWT Pratt, MJ Larkin: The Latent Heat of Vaporization and the Thermochemistry of Ethyl Nitrate. In: J. Chem. Soc. 1956, pp. 210-212, doi : 10.1039 / JR9560000210 .
- ↑ JA Dean (Ed.): Lange's Handbook of Chemistry. 15. Ed, McGraw-Hill, Inc. New York 1999, ISBN 0-07-016384-7 .
- ^ E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases . Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ Roth, L .; Weller, U .: Hazardous Chemical Reactions , 65th supplement, ecomed-Verlag 2011.
- ↑ Explosives Act, Appendix I, List of Explosive Substances ( BGBl. 1975 I p. 853 ), to which the law is to be applied in full.
- ↑ a b c d e f g J. Köhler, R. Meyer, and A. Homburg: Explosivstoffe , tenth, completely revised edition. Wiley-VCH, Weinheim 2008, p. 123, ISBN 978-3-527-32009-7 .


