Methyl nitrate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
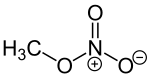
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methyl nitrate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | CH 3 NO 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 77.04 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.21 g cm −3 |
||||||||||||||||||
| Melting point |
−83.0 ° C |
||||||||||||||||||
| boiling point |
64.6 ° C |
||||||||||||||||||
| solubility |
little in water |
||||||||||||||||||
| Refractive index |
1.3748 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−156.3 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Methyl nitrate is a water-clear liquid with a strong odor and is obtained through the esterification of methanol .
Extraction and presentation
Methyl nitrate is an explosive that is produced by careful esterification of methanol with a nitric acid / sulfuric acid mixture, also known as nitrating acid , under strong cooling:
Accordingly, methyl nitrate is an ester of nitric acid with methanol as the alcoholic component. In addition, a mixture of 65% nitric acid with methanol with the addition of a little urea , which by binding nitrous gases , prevents decomposition and even an explosion, occurs even if it is carefully distilled off . Because of its sensitivity, methyl nitrate is of no practical use as an explosive .
properties
Methyl nitrate is a colorless liquid that smells like chloroform and boils at 66 ° C under normal pressure . The heat of vaporization at the boiling point is 28.673 kJ mol −1 . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in Torr, T in ° C) with A = 7.34608, B = 1351.0264 and C = 237.714 in the temperature range from -24.82 to 88.27 ° C. The compound has a density of 1.21 g · cm −3 and is extremely explosive. Methyl nitrate is a water-clear liquid with a strong, aromatic odor that quickly causes severe headaches. The impact sensitivity is lower than that of glycerin trinitrate (nitroglycerin) , but the volatility is significantly higher.
The explosive power is similar to nitroglycerin, the substance also gelatinizes together with nitrocellulose , so that Alfred Nobel also had the analogous mixture protected with methyl nitrate when describing the explosive gelatine, which was never used technically because of its volatility. The vapors of methyl nitrate are extremely explosive even without air access, which in the 19th century led to several catastrophic explosions during the temporary production of the substance as an intermediate product for dye synthesis (e.g. in St. Denis, 1874). The methyl nitrate burns off quietly in an open bowl without prior evaporation; if ignited in a test tube , it explodes without the need for an initial igniter . Important explosion indicators are:
- Heat of explosion : 6754 kJ kg −1 [H 2 O (l)] , 6055 kJ kg −1 [H 2 O (g)]
- Detonation velocity : 6300 m · s −1 at a density of 1.217 g · cm −3
- Normal gas volume : 909 l kg −1
- Specific energy : 1301 kJ kg −1
- Deflagration point : rapid evaporation without ignition
- Lead block expansion : 610 cm 3 / 10g
- Impact sensitivity : 0.2 N · m
- Sensitivity to friction : no reaction up to 353 N pin load
- Steel sleeve test : limit diameter 18 mm
use
Methyl nitrate did not attract much attention as an explosive, but as a mixture with a content of 25% methanol it was used as rocket fuel and volumetric explosive under the name Myrol in the Third Reich .
According to A. Stettbacher, the substance was used as fuel in the Reichstag fire in 1933 . Gartz shows in a recent work that only methyl nitrate, with its production and explosion potential, can represent the famous and mysterious “shooting water” from the German fireworks book from around 1420.
The text in the Fireworks Book of 1420 is in excerpts as follows:
"Wildu schyessen with water // that you don't have a pulfer // est vnd sterker and waiter // with you then as you that all // pest pulfer have got the yemann //
en mag and ye was made so ny // saltpeter and distill the with water // and nym oleo benedicto also ... // ... and zunt them with your senses that you may get away with it ... //
... with this water you wash three thousand feet far ... // ... it is delicious ... "
structure
The structure of methyl nitrate was investigated experimentally in the gas phase (combined gas-electron diffraction and microwave spectroscopy, GED / MW) and in the crystalline state (X-ray diffraction, XRD) (see Table 1).
In the solid body there are weak interactions between the O and N atoms of different molecules (see figure).
| parameter | ||
| XRD | GED / MW | |
| C-O | 1,451 (1) | 1,425 (3) |
| N-OC | 1,388 (1) | 1,403 (2) |
| N-O terminal | 1,204 (1) | 1,205 (1) |
| C-O-N | 113.3 (1) | 113.6 (3) |
| O terminal -NO terminal | 128.6 (1) | 131.4 (4) |
See also
Individual evidence
- ↑ a b Entry on nitric acid ester. In: Römpp Online . Georg Thieme Verlag, accessed on May 25, 2014.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-360.
- ↑ a b c d e f g h i j k l m Köhler, J .; Meyer, R .; Homburg, A .: Explosivstoffe , tenth, completely revised edition. Wiley-VCH, Weinheim 2008, ISBN 978-3-527-32009-7
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-20.
- ↑ RI Bialke: initiating explosives and ignition - syntheses of various primary explosives and the description of the preparation of the ignition , Books on Demand GmbH, Norderstedt ISBN 978-3-837-077827 , p. 37
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons , 1st Edition Elsevier 2008, ISBN 978-0815515968 , p. 411.
- ↑ Carl L. Yaws: The Yaws Handbook of Vapor Pressure - Antoine Coefficients , 2nd Edition Elsevier 2015, ISBN 978-0-12-802999-2 , p. 3, doi : 10.1016 / B978-0-12-802999-2.00004- 0 .
- ↑ Koch, E.-C .: Sprengstoffe, Treibmittel, Pyrotechnika , 2nd edition, de Gruyter, Berlin / Boston 2019, ISBN 978-3-11-055784-8 , p. 396, (accessed via De Gruyter Online).
- ^ A. Stettbacher: explosives and guns . Rascher Verlag, Zurich 1948.
- ↑ J. Gartz: From Greek fire to dynamite - a cultural history of explosives . ES Mittler & Sohn, Hamburg, 2007, ISBN 978-3-8132-0867-2 .
- ↑ Marco Reichel, Burkhard Krumm, Yury V. Vishnevskiy, Sebastian Blomeyer, Jan Schwabedissen, Marco Reichel, Hans ‐ Georg Stammler, Konstantin Karaghiosoff, Norbert W. Mitzel: Solid and gas phase structures and energetic properties of the dangerous methyl and fluoromethyl nitrate . In: Angewandte Chemie . tape 131 , no. 51 , 2019, ISSN 1521-3757 , p. 18730–18734 , doi : 10.1002 / anie.201911300 .


