Potassium formate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Potassium formate | ||||||||||||||||||
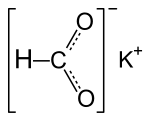
| Molecular formula | CHKO 2 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 84.12 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.91 g cm −3 |
||||||||||||||||||
| Melting point |
165-168 ° C |
||||||||||||||||||
| solubility |
very easy in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−679.7 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Potassium formate is the potassium salt of formic acid with the formula K (HCOO). It is a colorless, deliquescent substance in a crystalline , rhombic form. The density is 1.91 g / cm 3 , the melting point is 167.5 ° C. Potassium formate occurs as an intermediate in the now technically insignificant formate-potash process for the production of potassium carbonate . The part of the name -formiat goes back to the Latin word formica (ant).
Potassium formate is used as a surface de-icing agent , for example on roads and airports, and in a study by the Finnish Environment Institute (SYKE) has proven to be relatively environmentally friendly compared to de-icing agents that contain salt .
Extraction and presentation
Potassium formate can be produced from potassium hydroxide and formic acid by the salt formation reaction .
The synthesis from potassium carbonate and formic acid with the evolution of carbon dioxide is also possible.
Individual evidence
- ↑ a b c data sheet potassium formate from AlfaAesar, accessed on March 26, 2010 ( PDF )(JavaScript required) .
- ↑ a b c Entry on potassium formate in the GESTIS substance database of the IFA , accessed on May 9, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-19.
- ↑ Alternative de-icer found ( Memento of September 27, 2007 in the Internet Archive ), press release of the Finnish Ministry of the Environment of October 2, 2004.
- ↑ T. Meisel, Z. Halmos, K. Seybold, E. Pungor: "The thermal decomposition of alkali metal formats", in: Journal of Thermal Analysis and Calorimetry , 1975 , 7 (1), pp. 73-80 doi : 10.1007 / BF01911627

