Carbon-based Selective Catalytic Reduction
The carbon-based selective catalytic reduction ( CSCR for short , from the English carbon selective catalytic reduction ) describes a dry exhaust gas cleaning process to reduce pollutants in exhaust gases from large-scale plants such as in waste incineration plants for household and industrial waste , steelworks , coal-fired power plants , LCD glass production and in the Ammunition recycling.
The process is characterized by a single or multi-stage activated coke moving bed reactor, which is operated in countercurrent . Activated coke serves both as a catalyst for the oxidation of certain exhaust gas components and as a "storage" for adsorbed sulfur dioxide ( SO 2 ) in the form of sulfuric acid ( H 2 SO 4 ) or other pollutants . The loaded activated coke is discharged and, depending on the application, can be regenerated or burned in coal-fired power plants.
For denitrification , a nitrogen oxide ( NO x ) reducing gas, mostly ammonia ( NH 3 ), is added to the exhaust gas after prior SO 2 separation . Then NO x is converted in a further activated coke bed in the presence of activated coke as a catalyst together with NH 3 to form nitrogen ( N 2 ) and water ( H 2 O ).
In addition to nitrogen and sulfur oxides, due to the diverse properties of activated coke, other pollutants such as dioxins and furans , dust, heavy metals such as mercury and halogens such as hydrogen fluoride and hydrogen chloride are separated.
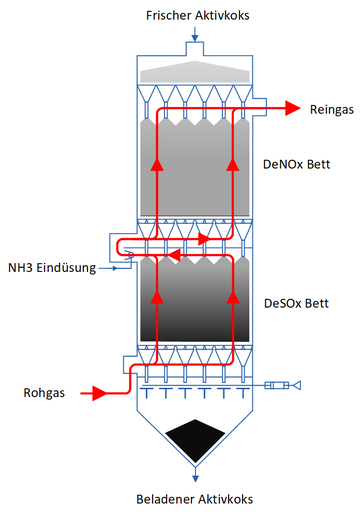
Structure and functionality of a CSCR reactor
The CSCR technology is an adsorptive and absorptive dry moving bed process . The flue gas flow is opposite to the adsorbent flow using the countercurrent principle .
The flue gas is led at a temperature of 90 ° C to 140 ° C into the flue gas collecting space below the activated coke bed. From there the gas enters an activated coke bed. If necessary, the first activated coke bed is followed by a second one.
The first bed is mostly used to separate sulfur oxides ( SO x ) and dust. The dust contained in the exhaust gas is separated due to the filter effect of the activated coke bed. The sulfur dioxide ( SO 2 ) contained in the exhaust gas is catalytically oxidized to sulfur trioxide ( SO 3 ) on the activated coke surface and converted with steam ( H 2 O ) to sulfuric acid ( H 2 SO 4 ), which is chemically absorbed in the pore system of the activated coke. In addition to SO 2 and dust, other pollutants are also adsorbed by the activated coke.
The denitrification takes place in the second bed . Before the flue gas reaches the second bed, it is fed into a mixing chamber in which gaseous ammonia water is added. With the added ammonia ( NH 3 ), the nitrogen oxides in the flue gas react on the catalytic surface of the activated coke to form the harmless products nitrogen ( N 2 ) and water. The flue gas leaves the second bed and can be cleaned and released into the ambient air via a chimney.
The structural design of the adsorber inflow base ensures, on the one hand, an even distribution of the flue gas when it enters the activated coke bed and, on the other hand, ensures that the bed sinks plane-parallel when the loaded activated coke is discharged.
When the discharge device is operated, the activated coke enters the discharge funnel. The activated coke bed is lowered by a few millimeters and fresh activated coke automatically slides out of the storage bunker into the adsorption area of the adsorber.
In comparison with other reactors, the countercurrent principle ensures that the activated coke is loaded evenly over the entire surface area. Another advantage of the countercurrent principle is the better activated coke loading capability and thus the minimization of activated coke consumption. The latter is achieved in that only the most heavily loaded activated coke layer is withdrawn in almost any adjustable batches via the simple, regulated extraction.
Activated coke can be regenerated with thermal treatment. In this way, a highly concentrated SO 2 -rich gas is obtained, which can be used, for example, to produce elemental sulfur or sulfuric acid.
Chemical reaction
According to the reaction equation shown, SO 2 contained in the exhaust gas is catalytically oxidized to SO 3 on the activated coke surface in the temperature range of about 20-150 ° C , which is converted into sulfuric acid with the water vapor in the exhaust gas.
A small part of the sulfuric acid formed is reacted with the basic ash components to form the corresponding sulfates .
Only after the DeSO x bed is NH 3 added to the flue gas as a reducing agent, since NH 3 would react with SO 2 first due to its affinity . This results in sulfates and bisulfates , which are deposited on the activated coke and inhibit its catalytic properties. For this reason, a large part of the SO 2 is separated out without NH 3 in the first activated coke bed .
In the second activated coke bed, which is intended for denitrification (DeNO x ), the remaining SO 2 that was not separated in the DeSO x bed then reacts with ammonia and water vapor to form sodium sulphate and is absorbed in the pore system.
The composition of the flue gas is determined by the fuel used and the combustion parameters, but the rule applies that nitrogen oxides from 5 - 10% vol. NO 2 and 90 - 95% vol. NO exist. The oxygen content of exhaust gases varies between 1% vol. for gas firing and about 15% vol. for waste incineration plants. Nitrogen and carbon dioxide (CO 2 ) are residues and do not take part in the separation reaction.
Nitrogen monoxide oxidizes catalytically on the surface of the activated coke and with the help of the oxygen in the exhaust gas to form nitrogen dioxide.
NO 2 is then reduced by the ammonia added in the mixing chamber and the products nitrogen and water vapor leave the system together with the cleaned flue gas.
Nitrogen monoxide can also be catalytically converted directly.
The main reaction for nitric oxide deposition is as follows.
The latter reaction only takes place in the presence of oxygen, which is contained in the exhaust gas. An increase in the oxygen concentration in the flue gas above 5% vol. however, it does not lead to a further increase in the NO reduction. Furthermore, excess NH 3 addition increases the conversion, but often leads to NH 3 slip, which has to be measured in the clean gas.
CSCR in the steel industry
Exhaust gases from sintering belt processes represent a significant proportion of the impurities that arise in steel production as a whole. Due to the very high amounts of exhaust gas that arise in sintering belt systems, a satisfactory exhaust gas cleaning is associated with high costs and great effort, since several cleaning steps have to be connected in series with conventional cleaning processes.
In particular, when nitrogen oxides are to be removed from the flue gas, the challenge is that other pollutant components such as SO 2 and HCl act like a catalyst poison on the activated coke .
With CSCR technology this problem is reduced or largely eliminated. With this method, SO 2 and NO x can be separated off at the same time or damage to the catalyst by performing the sinter exhaust gas cleaning process as a two-stage moving bed reactor. Even if the sinter exhaust gas still has significant concentrations of SO 2 and / or HCl after a pre-cleaning process, for example with a wet scrubber , these substances are separated in the first step of the CSCR process without burdening the NO x cleaning process and the catalyst used for this significant damage.
Preferred methods for pre-cleaning the sinter exhaust gas before it is transferred to the CSCR are hose or electrostatic precipitators, exhaust gas washers or entrained flow absorbers, for example with lime dust.
With this countercurrent moving bed process, residual values in the cleaned sintering exhaust gas of 10 mg / Nm 3 SO 2 , 50 mg / Nm 3 NO x and 10 mg / Nm 3 dust are achieved.
Due to the advantages that CSCR offers, this process has slipped more and more into the middle of a niche technology and is used in many steelworks in Asia, for example in plants of the Jiangsu Shagang Group , the Anshan Iron and Steel Group and the Masteel Group .
Individual evidence
- ^ WKV - Dr. Grochowski Anlagentechnik GmbH. Retrieved on March 26, 2020 (German).
- ↑ Dr. Horst Grochowski: PROCESS FOR CLEANING EXHAUST GASES FROM A GLASS MELTING PROCESS, IN PARTICULAR FOR GLASSES FOR LCD SCREENS. European publication server, 2011, accessed on March 19, 2020 (de / fr).
- ↑ Isao Mochida, Yozo Korai, Masuaki Shirahama, Shizuo Kawano, Tomohiro Hada: Removal of SOx and NOx over activated carbon fibers . In: Carbon . tape 38 , no. 2 , January 1, 2000, ISSN 0008-6223 , p. 227-239 , doi : 10.1016 / S0008-6223 (99) 00179-7 ( sciencedirect.com [accessed March 17, 2020]).
- ↑ CarboTech GmbH | Your specialist for activated carbon. Retrieved March 17, 2020 .
- ^ Margit Löschau: Cleaning of exhaust gases . TK-Verlag, Nietwerder 2014, ISBN 978-3-944310-13-8 , pp. 220-222 .
- ↑ Catalyst for reduction of nitrogen oxides in the presence of ammonia - Patent US4036785. Retrieved March 17, 2020 .
- ↑ Dr. Horst Grochowski: Inflow plate for moving bed reactors. May 5, 1988 ( google.com [accessed March 19, 2020]).
- ↑ Ekkehard Richter, Hans-Jürgen Schmidt, Hans-Georg Schecker: Adsorption and catalytic reactions of NO and NH3 on activated carbon . In: Chemical Engineering & Technology . tape 13 , no. 1 , 1990, ISSN 1521-4125 , pp. 332-340 , doi : 10.1002 / ceat.270130146 .
- ↑ Gomi, Kenichi; Komuro, Takeo; Arashi, Norio; Hishinuma, Yukio; Kanda, Osamu; Kuroda, Hiroshi: Reduction of nitrogen monoxide (NO) with ammonia in sulfur dioxide-containing gas on activated carbon and vanadium pentaoxide-loaded activated carbon. Ed .: Chemical Society of Japan. 4th edition. 1986, p. 527-531 .
- ↑ Karl Knoblauch, Ekkehard Richter, Harald Jüntgen: Application of active coke in processes of SO2 and NOx removal from flue gases . In: Fuel (= Industrial Conversion of Coal and Carbon to Gas, Liquid and High-Value Solid Products ). tape 60 , no. 9 , September 1, 1981, ISSN 0016-2361 , pp. 832-838 , doi : 10.1016 / 0016-2361 (81) 90146-0 .
- ↑ Dr. Horst Grochowski: Process for cleaning exhaust gases from a sintering process of ores and / or other metal-containing materials in metal production . February 8, 2006 ( google.com [accessed March 23, 2020]).