Copper germanat
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
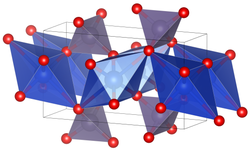
|
|||||||
| __ Cu 2+ __ Ge 4+ __ O 2− | |||||||
| Crystal system |
orthorhombic |
||||||
| Space group |
Pbmm (No. 51, position 3) |
||||||
| Lattice parameters |
a = 481 pm, b = 847 pm, c = 294.1 pm |
||||||
| Coordination numbers |
[6] Cu, [4] Ge |
||||||
| General | |||||||
| Surname | Copper germanat | ||||||
| other names |
|
||||||
| Ratio formula | CuGeO 3 | ||||||
| Brief description |
green-blue solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 184.1 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
1179–1200 ° C (decomposition) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Kupfergermanat is a chemical compound of the copper from the group of germanates .
Extraction and presentation
Copper germanate can be obtained by reacting copper or copper (II) oxide with germanium dioxide .
properties
Copper germanate is a green-blue solid. It has an orthorhombic crystal structure with the space group Pbmm (space group no. 51, position 3) . At high pressures there is a transition to a monoclinic crystal structure. The junction exhibits a specific magnetic behavior known as the Peierls transition at 14K. It was the first inorganic compound discovered to show this behavior.
Individual evidence
- ↑ a b H. Völlenkle, A. Wittmann, H. Nowotny: On the crystal structure of CuGeO 3 . In: Monthly magazine for chemistry . 98, 1967, pp. 1352-1357, doi : 10.1007 / BF00909002 .
- ↑ a b c Jane E. Macintyre: Dictionary of Inorganic Compounds, Supplement 3 . CRC Press, 1995, ISBN 978-0-412-49110-8 , pp. 279 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ LZ Pei, YQ Pei u. a .: Dependence of growth conditions on copper germanate nanowires and their electrochemical characteristics. In: Materials Science-Poland. 29, 2011, pp. 241-247, doi : 10.2478 / s13536-011-0040-6 .
- ↑ a b Makoto Tachibana: Beginner's Guide to Flux Crystal Growth . Springer, 2017, ISBN 978-4-431-56587-1 , pp. 104 ( limited preview in Google Book search).
- ^ High Pressure Science And Technology - Proceedings Of The Joint XV AIRAPT & XXXIII EHPRG International Conference . World Scientific, 1996, ISBN 981-4548-16-2 , pp. 423 ( limited preview in Google Book search).
- ↑ Kenneth R. O'Neal, Amal al-Wahish et al. a .: Charge and Bonding in CuGeO 3 Nanorods. In: Nano Letters . 18, 2018, pp. 3428-3434, doi : 10.1021 / acs.nanolett.8b00407 .
- ↑ Mark A. Green, Mohamedally Kurmoo u. A .: The crystal structure and magnetic properties of CuGeO 3 . In: Journal of the Chemical Society, Chemical Communications . 1994, pp. 1995-1996, doi : 10.1039 / C39940001995 .