Lithol red R
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
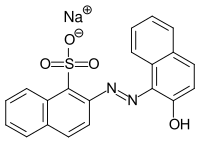
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Lithol red R | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| Brief description |
red solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 400.39 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| solubility |
Sodium salt hardly soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Lithol Red R is a chemical compound from the group of azo colorants . The metal salts ( sodium , barium , calcium and strontium ) are used as laked monoazo pigments (Pigment Red 49 types) in the printing industry .
Extraction and presentation
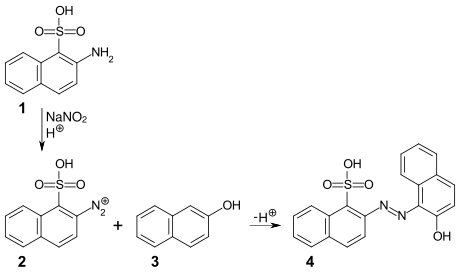
Lithol red R 4 can be synthesized by the diazotization of 2-aminonaphthalene-1-sulfonic acid (Tobias acid) 1 and subsequent azo coupling of the diazonium compound 2 with 2-naphthol 3 .
history
Lithol red was discovered at the end of the 19th century by the Austrian chemist Paul Julius , who worked for the German company BASF . To produce a new azo dye, Julius manipulated a cotton cloth impregnated with β-naphthol and diazotized Tobias acid . Julius reportedly was initially disappointed with his discovery because the new paint had a low affinity for cotton fibers, but further work convinced him of its potential as a pigment. He applied for a patent, which was granted in 1899.
Lithol Red R was the first technically important and synthetically manufactured commercial product from BASF from the group of azo pigments and was the starting point for BASF's systematic development of organic pigments.
In the 1950s it was the most important red pigment in the printing industry and was used here in greater quantities than any other red pigment. Last but not least, its inexpensive and uncomplicated production as well as the high gloss and good processing properties led to this supremacy. In the present day its importance has decreased increasingly. It is still used much more frequently in the United States than in Europe.
properties
The color of the sodium salt of Lithilrot R is yellowish red. The replacement of sodium by barium or strontium leads to a bright red, or calcium to a bluish-red hue. The light and solvent fastness is poor, as is the resistance to acids and alkalis. The laked pigments of this type do not have good heat resistance, but are relatively resistant to oils and fats. So it is called z. B. "Barium and calcium lithol red, which were exposed to the sun in Florida for a period of three months, have completely faded". The addition of titanium dioxide accelerates this whitening effect.
VIS spectroscopic investigations showed that the hydrazone form is preferred to the tautomeric azo form of the lithol red salts .
-
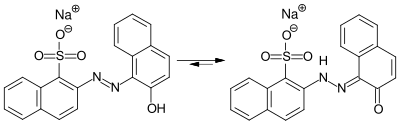
Azo-hydrazo-tautomerism in Litholrot R
use
The importance of the sodium salt of Lithol Red R as a pigment is rather low due to its significantly poorer application properties compared to the alkaline earth metal- coated products. Barium-lithol red has the largest share of commercial products. The importance of the Pigment Red 49 types varies greatly from region to region. In Europe and Japan they play a subordinate role, while in the USA they are used primarily as printing inks. Ultimately, however, due to the solubility of the free acid, the salts that can be produced from it by so-called laking are more likely to be used :
- More like a soluble dye than a pigment, which is rarely used due to its water solubility, which is too high for a pigment, and therefore bleeding when it comes into contact with water. It is used in cheap solvent-based flexographic printing inks and primarily as a starting material for the Ba, Ca and Sr laked pigments.
- Barium salt:
- Used as a water-insoluble pigment for water-based printing inks
- Calcium Salt & Strontium Salt:
- In the case of non-water-based uses, the choice of salts for the coloring depends primarily on the respective color effect, which differs with the different cations.
In the past, however, Lithol Red R was used much more widely - the Lithol pigments were included in the Color Index in 1971, with the following applications being named:
alkyd resin enamels and lacquers, linoleum, paper coating, emulsion paints, polyvinyl chloride, urea formaldehyde, phenol formaldehyde, polystyrene and amide-based plastics, and student grade artists' materials .
In the EU, the use of all lithol red salts in cosmetic products is currently permitted up to a mass fraction of 3%, while this use is completely prohibited in the USA.
proof
The method of pyrolysis - gas chromatography with coupled mass spectrometry (PYGCMS) has proven itself for the investigation of azo pigments, also for lithol red .
Individual evidence
- ↑ GG Sward: Paint Testing Manual . ASTM International, 1972, p. 512 ( limited preview in Google Book Search).
- ↑ External identifiers or database links for Pigment Red 49, free acid : CAS number: 29128-55-0, EC number: 249-459-0, ECHA InfoCard: 100.044.948 , PubChem : 14200 , ChemSpider : 21173279 , Wikidata : Q75829232 .
- ↑ External identifiers or database links for Pigment Red 49: 1, Ba salt : CAS number: 1103-38-4, EC number: 214-160-6, ECHA InfoCard: 100.012.873 , PubChem : 14199 , ChemSpider : 21171792 , Wikidata : Q75830061 .
- ↑ External identifiers of or database links for Pigment Red 49: 2, Ca salt : CAS number: 1103-39-5, EC number: 214-161-1, ECHA InfoCard: 100.012.874 , PubChem : 14201 , ChemSpider : 21171793 , Wikidata : Q75831378 .
- ↑ External identifiers or database links for Lithol Red Strontium : CAS number: 6371-67-1, EC number: 228-898-1, ECHA InfoCard: 100.026.271 , PubChem : 80751 , ChemSpider : 14678613 , Wikidata : Q27280751 .
- ^ A b Gisbert Otterstätter: Coloring of foods, medicines, cosmetics . Behr's Verlag DE, 2007, ISBN 3-89947-958-0 , p. 209 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Willy Herbst, Klaus Hunger: Industrial organic pigments . Production, properties, application. VCH Verlagsgesellschaft, Weinheim 1995, ISBN 3-527-28744-2 , pp. 326 ff . ( limited preview in Google Book search).
- ↑ a b c Harriet AL Standeven: The History and Manufacture of Lithol Red, a Pigment Used by Mark Rothko in his Seagram and Harvard Murals of the 1950s and 1960s . In: TATE (ed.): Tate Papers . No. 10 . London 2008.
- ^ Werner Abelshauser: The BASF a company history . CH Beck, 2002, ISBN 978-3-406-49526-7 , pp. 70 ( limited preview in Google Book search).
- ↑ Prof. Dr. Artur Goldschmidt, Dr. Hans-Joachim Streitberger: BASF Handbook - Painting Technology . Ed .: BASF Coatings AG. Vincentz Verlag, Hannover 2002, ISBN 3-87870-324-4 , p. 153 ( limited preview in Google Book search).
- ^ CH Beck: The BASF - A company history . Ed .: Werner Abelshauser. 2nd Edition. CH Beck oHG, Munich 2003, ISBN 3-406-49526-5 , p. 70 .
- ^ AWC Harrison: The Manufacture of Lakes and Precipitated Pigments . Ed .: Leonard Hill Limited. London 1957, p. 232 .
- ^ Robert Leach, Ray Pierce: The Printing Ink Manual . Springer Science & Business Media, 2007, ISBN 978-1-4020-6187-5 , pp. 159 ( limited preview in Google Book search).
- ^ Vincent C. Vesce: Exposure Studies of Organic Pigments in Paint Systems - Joseph J. Mattiello Memorial Lecture . Ed .: Federation of Societies for Paint Technology. Allied Chemical, National Aniline Division, Atlantic City, New Jersey 1959.
- ^ Wojciech Czajkowski: Spectral studies of lithol red pigments . In: Dyes and Pigments . tape 8 , no. 2 , p. 141-150 , doi : 10.1016 / 0143-7208 (87) 85012-x ( elsevier.com ).
- ↑ Werner Baumann, Bettina Herberg-Liedtke: Printing chemicals data and facts on environmental protection . Springer-Verlag, 2013, ISBN 978-3-642-97337-6 , pp. 951 ( limited preview in Google Book Search).
- ^ Society of Dyers and Colourists & AATCC (eds.): Color Index . 1971, p. 3307 .
- ↑ Jens Stenger et al .: Lithol red salts: characterization and deterioration . In: Morana RTD doo (ed.): E-Preservation Science . No. 7 , December 1, 2010, p. 147-157 .
- ↑ Astrid Rehorek, Alexander Plum: Characterization of sulfonated azo dyes and aromatic amines by pyrolysis gas chromatography / mass spectrometry . In: Analytical and Bioanalytical Chemistry . tape 388 , no. 8 , August 1, 2007, p. 1653–1662 , doi : 10.1007 / s00216-007-1390-0 .