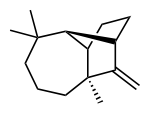
Longifolen
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Longifolen | |||||||||||||||
| other names |
(1 R , 2 S , 7 S , 9 S ) -3,3,7-trimethyl-8-methylentricyclo- [5.4.0.0 2.9 ] undecane |
|||||||||||||||
| Molecular formula | C 15 H 24 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 204.36 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.9328 g cm −3 |
|||||||||||||||
| boiling point |
260-261 ° C |
|||||||||||||||
| Refractive index |
1.504 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Longifolen is the common name of a naturally occurring hydrocarbon . Longifolen is a chiral , dextrorotatory , tricyclic sesquiterpene .
Occurrence
The name is derived from Pinus longifolia , a name that is no longer in use for the pine Pinus roxburghii , from whose resin it was isolated and in which it is present in a concentration of 5 to 10%.
use
Longifolen is used in organic chemistry for the production of dilongifolylborane, a chiral hydroboration reagent .
Longifolen is one of the main aromatic components of the tea specialty Lapsang Souchong , as it is smoked over pine wood.
synthesis
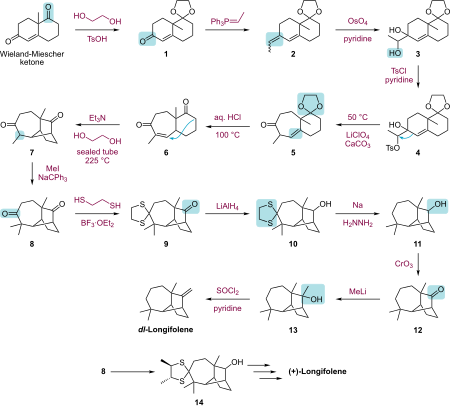
The structure of the tricyclic compound has been investigated by various research groups. The total synthesis of EJ Corey led to the development of new synthetic methods.
The synthesis according to Corey takes place starting from the Wieland-Miescher ketone in a thirteen-step synthesis via Wittig reaction , oxidation of the resulting double bond to the diol by means of osmium tetroxide , ring expansion , internal cyclization and further reaction steps with formation of the racemate .
The preparation of the optically active component is achieved in a multistage synthesis using (+) - 2,3-butanedithiol as a chiral agent.
Individual evidence
- ↑ a b c Data sheet (+) - Longifolene from Sigma-Aldrich , accessed on December 28, 2013 ( PDF ).
- ^ N. Mirov: Composition of Gum Turpentines of Pines , 1961, US Forest Service, Technical Bulletin No. 1239, US DEPT OF AGRICULTURE, p. 57.
- ^ N. Mirov: Composition of Gum Turpentines of Pines , 1961, US Forest Service, Technical Bulletin No. 1239, US DEPT OF AGRICULTURE, p. 18.
- ↑ a b Entry on (+) - Longifolen in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ^ John Lionel Simonsen: LXI. The constituents of Indian turpentine from Pinus longifolia, Roxb. Part I. In: Journal of the Chemical Society, Transactions. 117, 1920, p. 570, doi : 10.1039 / CT9201700570 .
- ↑ P. Naffa, G. Ourisson: Le Longifolene. 3. Addition of the Hydracides Halogenes sur le Longifolene - Les Halogenures de Longibornyle-Produits Disomerisation du Longifolene. In: Bulletin de la Societe Chimique de France , 21.11-1 (1954), pp. 1410-1415.
- ↑ Suhk Dev: The chemistry of longifolene and its derivatives. In: Progress in the Chemistry of Organic Natural Products / Progress in the Chemistry of Organic Natural Products , 1981, Springer Verlag, Vienna, ISBN 978-3-7091-8613-8 , pp. 49-104.
- ↑ Prabhakar K. Jadhav, Herbert C. Brown: Dilongifolylborane: a new effective chiral hydroborating agent with intermediate steric requirements. In: The Journal of Organic Chemistry. 46, 1981, pp. 2988-2990, doi : 10.1021 / jo00327a036 .
- ↑ Shan-Shan Yao, Wen-Fei Guo, Y. i. Lu, Yuan-Xun Jiang: Flavor Characteristics of Lapsang Souchong and Smoked Lapsang Souchong, a Special Chinese Black Tea with Pine Smoking Process. In: Journal of Agricultural and Food Chemistry. 53, 2005, pp. 8688-8693, doi : 10.1021 / jf058059i .
- ↑ a b E.J. Corey, Masaji. Ohno, Rajat B. Mitra, Paul A. Vatakencherry: Total Synthesis of Longifolene. In: Journal of the American Chemical Society . 86, 1964, pp. 478-485, doi : 10.1021 / ja01057a039 .
- ↑ Eric J. Kantorowski, Mark J. Kurth: Expansion to Seven-Membered Rings . In: Tetrahedron . 56, No. 26, June 2000, pp. 4317-4353. doi : 10.1016 / S0040-4020 (00) 00218-0 .