Malondialdehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
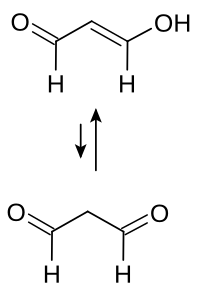
|
||||||||||||||||
| Tautomeric boundary structures of malondialdehyde | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Malondialdehyde | |||||||||||||||
| other names |
Propandial |
|||||||||||||||
| Molecular formula | C 3 H 4 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 72.06 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
72 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Malondialdehyde (MDA), according to IUPAC Propandial , is a highly reactive dialdehyde . It arises biochemically as a breakdown product of polyunsaturated fatty acids and is an important biomarker for oxidative stress .
properties
Pure malondialdehyde is unstable. It is usually commercially available as a dilute aqueous solution of the sodium salt of enol . In the laboratory, malondialdehyde can be generated in situ by hydrolyzing 1,1,3,3-tetramethoxypropane . Of the two possible enol isomers, the trans isomer is preferred in aqueous and the cis isomer in organic solvents.
Biological and biochemical importance
Malondialdehyde is an important breakdown product in the oxidation of polyunsaturated fatty acids. It serves as a biomarker for oxidative stress. For this, the solution to be tested, such as blood plasma , with thiobarbituric acid is added. One molecule of malondialdehyde reacts with two molecules of thiobarbituric acid to form a pink dye, which has an absorption maximum at 532 nm and can be determined photometrically or with the aid of high-performance liquid chromatography (HPLC).
Another reagent for the quantitative determination of malondialdehyde is a hydrochloric acid alcoholic solution of 2-methylindole . The red dye formed in the reaction with MDA has an absorption maximum at 555 nm.
In addition, the highly reactive malondialdehyde, which can react with DNA and proteins , is potentially mutagenic , atherogenic and carcinogenic . Its bifunctionality enables it to cross-link proteins, for example.
- Protein cross-link through the addition of malondialdehyde to protein amino groups (PNH 2 ).
The plasma MDA levels are significantly increased in patients with diabetes mellitus .
further reading
- J. Lykkesfeldt: Malondialdehyde as a biomarker of oxidative damage to lipids caused by smoking. In: Clinica chimica acta ; international journal of clinical chemistry Volume 380, number 1–2, May 2007, pp. 50–58, doi : 10.1016 / j.cca.2007.01.028 . PMID 17336279 .
- K. Uchida: Lipofuscin-like fluorophores originated from malondialdehyde. In: Free radical research Volume 40, Number 12, December 2006, pp. 1335-1338, doi : 10.1080 / 10715760600902302 . PMID 17090422 .
- DE Shuker, W. Atkin, SA Bingham, C. Leuratti, R. Singh: Malondialdehyde-DNA adducts in relation to diet and disease risk - a brief overview of recent results. In: IARC scientific publications Volume 156, 2002, pp. 475-480, PMID 12484237 .
- M. Dib, C. Garrel, A. Favier, V. Robin, C. Desnuelle: Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? In: Journal of neurology Volume 249, Number 4, April 2002, pp. 367-374, doi : 10.1007 / s004150200025 . PMID 11967639 .
- ME Haberland, D. Fong, L. Cheng: Malondialdehyde, modified lipoproteins, and atherosclerosis. In: European Heart Journal Volume 11 Suppl E, August 1990, pp. 100-104, PMID 2226517 .
Individual evidence
- ↑ a b Malonaldehyde. NIOSH Pocket Guide to Chemical Hazards, accessed June 13, 2011
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ V. Nair, CL O'Neil, PG Wang: Malondialdehyde. In: Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, New York, 2008 ( doi : 10.1002 / 047084289X.rm013.pub2 ).
- ↑ R. Ebermann, I. Elmadfa: Textbook food chemistry and nutrition. Verlag Springer, 2008, ISBN 3-211-48649-6 , p. 99. Restricted preview in the Google book search
- ↑ D. Ganten, K. Ruckpaul: Molecular medicine basics of para- and autocrine regulation disorders. Verlag Springer, 2006, ISBN 3-540-28781-7 , p. 174. Restricted preview in the Google book search
- ↑ H. Scherz, G. Stehlik, E. Bancher, K. Kaindl: 2-methylindole as a reagent on malondialdehyde. In: Microchimica Acta Volume 55, Number 5, 1967, pp. 915-919. doi : 10.1007 / BF01216836
- ↑ LJ Marnett: Chemistry and biology of DNA damage by malondialdehyde. In: IARC scientific publications number 150, 1999, pp. 17-27, PMID 10626205 .
- ↑ D. Del Rio, AJ Stewart, N. Pellegrini: A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. In: Nutrition, metabolism, and cardiovascular diseases Volume 15, Number 4, August 2005, pp. 316-328, doi : 10.1016 / j.numecd.2005.05.003 . PMID 16054557 .
- ↑ LJ Marnett: lipid peroxidation DNA damage by malondialdehyde. In: Mutation Research Volume 424, Number 1-2, March 1999, pp. 83-95, PMID 10064852 .
- ↑ C. Lehmann: Animal studies on the intestinal microcirculation in endotoxinemia. Habilitation thesis, Humboldt University Berlin, 2000.
- ↑ BS Berlett and ER Stadtman: Protein oxidation in aging, disease, and oxidative stress. In: J Biol Chem 272, 1997, pp. 20313-20316. PMID 9252331 .
- ↑ DA Slatter, CH Bolton, AJ Bailey: The importance of lipid-derived malondialdehyde in diabetes mellitus. In: Diabetologia Volume 43, Number 5, May 2000, pp. 550-557, doi : 10.1007 / s001250051342 . PMID 10855528 .

