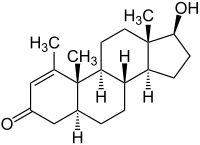
Methenolone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Methenolone | ||||||||||||||||||
| other names |
( 5α , 17β ) -17-Hydroxy-1-methylandrost-1-en-3-one |
||||||||||||||||||
| Molecular formula | C 20 H 30 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 302.45 g · mol -1 | ||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Methenolone , also called metenolone , is an organic chemical compound from the group of steroids . As a medicinal substance Primobolan ® , it shows a low androgenic , but strong anabolic effect.
Methenelon is either injected intramuscularly as methenolone enanthate ( Primobolan Depot ) or taken orally as methenolone acetate ( Primobolan S ). The steroid is quickly and completely absorbed after oral intake; after about 3.5 hours, 90% are metabolized to conjugates . The plasma half-life is about one day. Half of it is eliminated in the urine and half in the stool. The esters methenolone acetate and methenolone enanthate are more effective because they - depending on the tissue - first have to be converted into methenolone and the corresponding acid. The androgenic effect corresponds functionally to that of dihydrotestosterone ( androstanolone ).
Among the side effects include prostate growth , scalp hair loss, hair growth on the body, acne and masculinization in women through clitoral growth , beard growth and voice deepening.
Primobolan was manufactured by Schering AG in Germany and Mexico and is now manufactured by Bayer AG . Its use was widespread in bodybuilding circles in the 1970s .
Web links
- Entry on Methenolone at Vetpharm, accessed August 4, 2012.
Individual evidence
- ↑ a b C. R. Ganellin, David J. Triggle: Dictionary of pharmacological agents, Volume 2: H-Z. CRC Press, 1996, ISBN 978-0-412-46630-4 , p. 1293.
- ↑ There is not yet a harmonized classification for this substance . A labeling of Metenolone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on April 3, 2020, is reproduced from a self-classification by the distributor .
- ↑ a b F. v. Bruchhausen, S. Ebel, AW Frahm, E. Hackenthal: Hagers Handbook of Pharmaceutical Practice. Volume 8. Substances E-O. 5th edition, Springer, 1995, ISBN 978-3-540-52688-9 , pp. 907-908.
- ↑ Bernhard Kleine, Winfried Rossmanith: Hormones and hormone system: an endocrinology for bioscientists. Springer, 2007, ISBN 978-3-540-37702-3 , p. 229.
- ↑ Steve Theunissen: Arnold & Steroids: Truth Revealed ( Memento December 7, 2003 in the Internet Archive ), 2002.