Molybdenum (VI) oxide tetrachloride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Mo 6+ __ Cl - __ O 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Molybdenum (VI) oxide tetrachloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | MoOCl 4 | ||||||||||||||||||
| Brief description |
green solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 253.75 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
100 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Molybdenum (VI) oxide tetrachloride is an inorganic chemical compound of molybdenum from the group of oxide chlorides .
Extraction and presentation
Molybdenum (VI) oxide tetrachloride can be obtained by reacting molybdenum (VI) oxide with thionyl chloride .
An alternative representation is the reaction of molybdenum (V) chloride with oxygen at 215 to 220 ° C:
properties
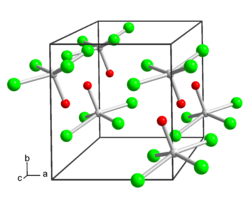
Molybdenum (VI) oxide tetrachloride is a dark green, diamagnetic , extremely moisture- and light-sensitive solid. It reacts with chlorobenzene to form molybdenum (V) oxide trichloride . Under nitrogen, it evaporates at 102 ° C to a brown vapor. It has a tetragonal crystal structure of the WOCl 4 type.
use
Molybdenum (VI) oxide tetrachloride is used as the basis for catalysts for the polymerization of substituted acetylenes .
Individual evidence
- ↑ a b c d e data sheet Molybdenum (VI) tetrachloride oxide, 97% from Sigma-Aldrich , accessed on July 6, 2013 ( PDF ).
- ↑ a b c Alan K. Mallock: Molybdenum (VI) oxide chloride . In: Earl L. Muetterties (Ed.): Inorganic Syntheses . tape 10 . McGraw-Hill Book Company, Inc., 1967, p. 52-57 (English).
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1539.


![{\ displaystyle {\ ce {2 MoCl5 + 2 O2 -> [T] [] 2 MoOCl4 + O2 + Cl2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4c0e0316aa0a16b262c485084ae0d8069c2e9f7a)