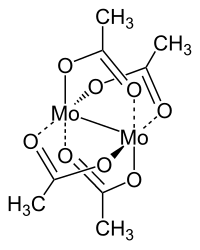
Molybdenum acetate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Molybdenum acetate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 12 Mo 2 O 8 | |||||||||||||||
| Brief description |
yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 428.079 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Molybdenum acetate is a chemical compound of molybdenum from the group of acetates with the dimeric constitutional formula [Mo (CH 3 COO) 2 ] 2 .
Extraction and presentation
Molybdenum acetate can be obtained by reacting molybdenum hexacarbonyl with an excess of glacial acetic acid to which a small amount of acetic anhydride has been added.
properties
Molybdenum acetate is a diamagnetic, flammable solid that is in the form of yellow crystal needles. It is practically insoluble in water and insoluble in practically all common solvents. Slow decomposition takes place at room temperature even under protective gas. It is extremely sensitive to moisture and forms a green hydrate. The connection has a triclinic crystal structure with space group P 1 (space group no. 2) .
Individual evidence
- ↑ a b c d e Entry for CAS no. 14221-06-8 in the GESTIS substance database of the IFA , accessed on July 7, 2013(JavaScript required) .
- ↑ a b c Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1554.
- ↑ D. Lawton, R. Mason: The Molecular Structure of Molybdenum (II) Acetate. In: Journal of the American Chemical Society. 87, 1965, pp. 921-922, doi : 10.1021 / ja01082a046 .

![{\ displaystyle \ mathrm {[Mon (CH_ {3} COO) _ {2}] _ {2} +12 \ CO + 2 \ H_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6f4df8c44e1c2eebde29deddf4c2b6449b4e398e)