Letter acids
Amine- and / or hydroxy-substituted naphthalene sulfonic acids are referred to as letter acids . They are important intermediates in dye chemistry . The name letter acid is derived from the fact that these acids are usually only named with a preceding letter, for example "H-acid". The letters were assigned historically and the choice of letter is not always comprehensible. Some acids are also labeled with the name of their discoverer (for example "Brönner acid") or bear the name of the company that first made this compound (for example "Cassella acid"); The last two types are also counted among the letter acids. The letter acids are used as diazo or coupling components in azo couplings or as substituents (for example to substitute a chlorine atom in cyanuric chloride ).
Naphthylamine sulfonic acids
One or more naphthylamine sulfonic acids are obtained from the naphthylamines α-naphthylamine and β-naphthylamine , depending on the reaction conditions, by sulfonation . More highly aminated compounds can be obtained by nitration and subsequent Béchamp reduction .
Naphthol sulfonic acids
One or more naphthol sulfonic acids are obtained from naphthols by sulfonation, depending on the reaction conditions. Aminated compounds can be obtained by nitration and subsequent Bechamp reduction.
Aminohydroxy naphthalene sulphonic acids
see aminohydroxynaphthalenesulfonic acids
nomenclature
The compounds have long been used as intermediates in the manufacture of azo dyes . Since the systematic names are confusing, most of them have been given trivial names. The salts of the sulphonic acids ( sulphonates ) are usually named after the "stem-trivial name": H- acid → H- salt , possibly also the cation ( sodium - H -salt). The trivial nomenclature is made even more confusing by the numbering of the substituents. Historically and for reasons of manufacturing technology, the carbon atom that carries the hydroxyl group and the one that carries the amino group is inconsistently given the locant 1 .
The trivial nomenclature was mainly created in the German-speaking area. In English , the translation is usually 1: 1 ( Brönner acid → Broenner's acid , also Brönner's acid ).
Examples
| Short name | Full name ( IUPAC ) | CAS No .: | structure | Remarks |
|---|---|---|---|---|
| H-acid | 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid | 90-20-0 |
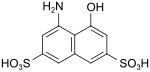
|
very important as a coupling component for red (monoazo) and blue (bisazo) dyes |
|
I-acid (name of I so-γ-acid) |
7-amino-4-hydroxynaphthalene-2-sulfonic acid | 87-02-5 |

|
very important as a coupling component for scarlet-red (monoazo) and red-brown (bisazo) dyes |
| γ-acid | 6-amino-4-hydroxynaphthalene-2-sulfonic acid | 90-51-7 |

|
important for red monoazo dyes which are lightfast as coupling components |
| K acid | 4-amino-5-hydroxynaphthalene-1,7-disulfonic acid | 130-23-4 |
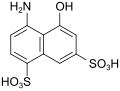
|
as a coupling component for red, lightfast monoazo dyes |
|
Tobias acid (also A acid) |
2-aminonaphthalene-1-sulfonic acid | 81-16-3 |

|
as a diazo component |
| Sulfo-Tobias acid | 2-aminonaphthalene-1,5-disulfonic acid | 117-62-4 |
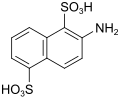
|
as a diazo component |
|
Broenner acid (also Brönner acid, Amino-Schäffer acid) |
6-aminonaphthalene-2-sulfonic acid | 93-00-5 |
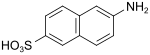
|
|
|
Schäffer acid (α) (also α-Schäffer acid, tree acid) |
1-hydroxynaphthalene-2-sulfonic acid | 567-18-0 |

|
|
|
C acid (also Cassella acid) |
3-aminonaphthalene-1,5-disulfonic acid | 131-27-1 |

|
as a diazo and coupling component |
|
Croceic acid (also Bayer acid, Rumpff acid) |
7-hydroxynaphthalene-1-sulfonic acid | 132-57-0 |

|
|
| Neville Winther Acid | 4-hydroxynaphthalene-1-sulfonic acid | 84-87-7 |

|
|
|
L-acid (also azuric acid, oxylaurent acid) |
5-hydroxynaphthalene-1-sulfonic acid | 117-59-9 |

|
|
| Schäffer acid (β) | 6-hydroxynaphthalene-2-sulfonic acid | 93-01-6 |

|
|
|
F acid (also Cassella acid) |
7-hydroxynaphthalene-2-sulfonic acid | 92-40-0 |
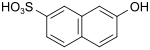
|
|
| G acid | 7-hydroxynaphthalene-1,3-disulfonic acid | 118-32-1 |
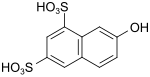
|
|
|
δ acid (also CS acid, Schöllkopf acid, oxy-Chicago acid) |
4-hydroxynaphthalene-1,5-disulfonic acid | 117-56-6 |
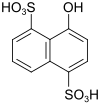
|
|
|
D-acid (double name, see 81-05-0!) |
4-hydroxynaphthalene-1,6-disulfonic acid | 6361-37-1 |

|
|
|
ε-acid (also Andresen acid) |
8-hydroxynaphthalene-1,6-disulfonic acid | 117-43-1 |

|
|
| R-acid | 3-hydroxynaphthalene-2,7-disulfonic acid | 148-75-4 |

|
|
|
RG acid (also GR acid) |
4-hydroxynaphthalene-2,7-disulfonic acid | 578-85-8 |

|
|
|
Boeniger acid (also 1,2,4 acid) |
4-amino-3-hydroxynaphthalene-1-sulfonic acid | 578-85-8 |

|
|
|
S acid (also Chicago S acid) |
4-amino-5-hydroxynaphthalene-1-sulfonic acid | 83-64-7 |
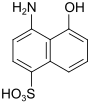
|
|
| M acid | 8-amino-4-hydroxynaphthalene-2-sulfonic acid | 489-78-1 |
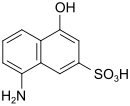
|
|
|
SS acid (also Chicago SS acid) |
4-amino-5-hydroxynaphthalene-1,3-disulfonic acid | 82-47-3 |
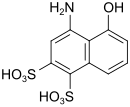
|
|
|
Sulfo-I-acid (also I-di-acid) |
2-amino-5-hydroxynaphthalene-1,7-disulfonic acid | 6535-70-2 |

|
as a coupling component |
|
RR acid (also 2R acid, Columbia acid) |
3-amino-5-hydroxynaphthalene-2,7-disulfonic acid | 90-40-4 |

|
|
|
Naphthionic acid (also piria acid) |
4-aminonaphthalene-1-sulfonic acid | 84-86-6 |
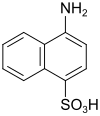
|
|
|
Purpuric acid (also laurent acid) |
5-aminonaphthalene-1-sulfonic acid | 84-89-9 |

|
|
|
Dahl acid (also Dahl acid I, D acid (double name, see 6361-37-1!)) |
6-aminonaphthalene-1-sulfonic acid | 81-05-0 |
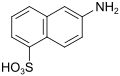
|
|
|
Badische Acid (also Forsling Acid I) |
7-aminonaphthalene-1-sulfonic acid | 86-60-2 |

|
|
|
Peric acid (also Forsling acid I) |
8-aminonaphthalene-1-sulfonic acid | 82-75-7 |

|
as a coupling component |
|
Cleve γ acid (also Cleve acid) |
4-aminonaphthalene-2-sulfonic acid | 134-54-3 |

|
|
|
Cleve-β-acid (also 1,6-Cleve-acid, μ-acid) |
5-aminonaphthalene-2-sulfonic acid | 119-79-9 |
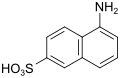
|
|
|
Cassella F acid (also δ acid, F acid (double name, see 92-40-0!), Bayer acid (?)) |
7-aminonaphthalene-2-sulfonic acid | 494-44-0 |

|
|
| 1,7 Cleve Acid | 8-aminonaphthalene-2-sulfonic acid | 119-28-8 |

|
|
|
Amino-I-acid (also Amino-J-acid) |
6-aminonaphthalene-1,3-disulfonic acid | 118-33-2 |

|
|
| Amino-G acid | 7-aminonaphthalene-1,3-disulfonic acid | 86-65-7 |

|
|
|
Amino-S-acid (also disulfo-S-acid) |
4-aminonaphthalene-1,5-disulfonic acid | 117-55-5 |
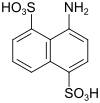
|
|
| Dahl acid III | 4-aminonaphthalene-1,6-disulfonic acid | 85-75-6 |

|
|
| Amino-ε-acid | 8-aminonaphthalene-1,6-disulfonic acid | 129-91-9 |

|
|
| Dahl acid II | 4-aminonaphthalene-1,7-disulfonic acid | 85-74-5 |

|
|
|
Alenic acid (also Freund's acid (1,3,7)) 1) |
4-aminonaphthalene-2,6-disulfonic acid | 6362-05-6 |

|
|
| Kalle acid | 1-aminonaphthalene-2,7-disulfonic acid | 486-54-4 |
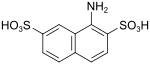
|
|
| Amino-R acid | 3-aminonaphthalene-2,7-disulfonic acid | 92-28-4 |

|
|
| Friend Acid (1,3,6) 1) | 4-aminonaphthalene-2,7-disulfonic acid | 6251-07-6 |
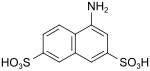
|
|
|
B acid (also melan acid) |
8-aminonaphthalene-1,3,5-trisulfonic acid | 17894-99-4 |
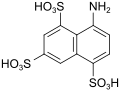
|
|
| 2R amino acid | 7-aminonaphthalene-1,3,6-trisulfonic acid | 118-03-6 |

|
|
|
T acid (also Koch acid, amino H acid) |
8-aminonaphthalene-1,3,6-trisulfonic acid | 117-42-0 |
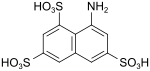
|
not identical to 2,4,5-trichlorophenoxyacetic acid (also called T-acid ) |
1) named after Louis Freund.
swell
- ↑ Entry on letter acids. In: Römpp Online . Georg Thieme Verlag, accessed on May 7, 2014.