Naphthylene-1,5-diisocyanate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
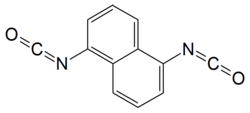
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Naphthylene-1,5-diisocyanate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 6 N 2 O 2 | |||||||||||||||
| Brief description |
Hardly flammable flakes, white to yellowish solid with an aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 210.19 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.42 g cm −3 |
|||||||||||||||
| Melting point |
127 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
<0.9 hPa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Naphthylene-1,5-diisocyanate is a chemical compound from the group of organic diisocyanates .
Extraction and presentation
Naphthylene-1,5-diisocyanate is produced industrially in high yields by pyrolysis of methyl-1,5-naphthylene carbamate , which in turn is synthesized from 1,5-naphthylenedinitrile and amidation , chlorination and Hofmann rearrangement .
properties
Naphthylene-1,5-diisocyanate is a flammable, hardly ignitable, white to yellowish solid with an aromatic odor, which is practically insoluble in water and slowly decomposes in it. The compound begins to decompose from 200 ° C.
use
Naphthylene-1,5-diisocyanate is mainly used in the automotive industry for the production of polyurethane - elastomer and synthetic rubber used. In its pure and technical form, it is a solid substance whose effects are comparable to those of diphenylmethane-4,4'-diisocyanate .
safety instructions
Naphthylene-1,5-diisocyanate has a strong irritant effect on the skin and mucous membranes. The metabolite of 1,5-naphthylene diisocyanate, 1,5-diaminonaphthalene , is genotoxic and carcinogenic in animal experiments. Since no data were available on the extent of hydrolytic formation of 1,5-diaminonaphthalene after ingestion of 1,5-naphthylene diisocyanate, 1,5-naphthylene diisocyanate was classified in carcinogen category 3B.
Individual evidence
- ↑ a b c d e f g h i j k Entry on naphthylene-1,5-diisocyanate in the GESTIS substance database of the IFA , accessed on January 28, 2019(JavaScript required) .
- ↑ a b c Leng, G. (2012): 1,5-Naphthylenediisocyanat (NDI), BAT Value Documentation , 2009, Annual Thresholds and Classifications for the Workplace, doi: 10.1002 / 3527600418.bb317372d0015 .
- ↑ Entry on 1,5-naphthylene diisocyanate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 28, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Google Patents: DE10034226A1 - Process for the production of 1,5-naphthylene diisocyanate - Google Patents , accessed on January 28, 2019

