Native chemical ligation
Native chemical ligation is a biochemical method of protein ligation by linking two or more peptides .
properties
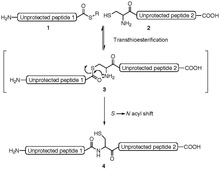
Protein ligation allows two peptides to be linked to one another. The N -terminal peptide must for protein ligation as the last amino acid (at its C -terminus ) a thioester and the C -terminal peptide has for protein ligation as first amino acid (at its N -terminus a) cysteine have. In the course of the ligation, a thioester bond is formed on the cysteine, which transesterifies and then rearranges to form a peptide bond (thiotransesterification and S, N-acyl shift ). The reaction takes place in aqueous solution in the presence of the chaotrope guanidinium chloride or urea . Any amino acid can be used as the thioester, but valine , isoleucine and proline are sterically hindered, which makes the reaction slower. The C -terminal thioester of the N -terminal peptide is mostly produced by peptide synthesis with Boc - protective groups . Since thioester-containing peptides cannot be generated with Fmoc protective groups and nucleophilic bases, a connoisseur safety catch linker is used to generate the thioester with Fmoc protective groups . Thiophenyl, 4-mercaptophenylacetic acid (MPAA) or 2-mercaptoethanesulfonate (MESNa) are used as the catalyst for the reaction.
Alternative methods of protein ligation have been described, e.g. B. Prior Thiol Capture , Expressed Protein Ligation , Acyl-Initiated Capture and Peptide Ligation with Selenocysteine .
history
The underlying reaction of native chemical ligation was published in 1953 by Theodor Wieland . From 1994 the reaction for the production of proteins from peptides was further developed by SB Kent .
Individual evidence
- ↑ TW Muir, PE Dawson, SB Kent: Protein synthesis by chemical ligation of unprotected peptides in aqueous solution. In: Methods in enzymology. Volume 289, 1997, ISSN 0076-6879 , pp. 266-298, PMID 9353726 .
- ↑ TM Hackeng, JH Griffin, PE Dawson: Protein synthesis by native chemical ligation: expanded scope by using straightforward methodology. In: Proceedings of the National Academy of Sciences . Volume 96, Number 18, August 1999, ISSN 0027-8424 , pp. 10068-10073, PMID 10468563 , PMC 17843 (free full text).
- ↑ TW Muir, D. Sondhi, PA Cole: Expressed protein ligation: a general method for protein engineering. In: Proceedings of the National Academy of Sciences . Volume 95, Number 12, June 1998, ISSN 0027-8424 , pp. 6705-6710, PMID 9618476 , PMC 22605 (free full text).
- ↑ BL Nilsson, MB Soellner, RT Raines: Chemical synthesis of proteins. In: Annual review of biophysics and biomolecular structure. Volume 34, 2005, ISSN 1056-8700 , pp. 91-118, doi: 10.1146 / annurev.biophys.34.040204.144700 , PMID 15869385 , PMC 2845543 (free full text).
- ^ T. Wieland, E. Bokelmann, L. Bauer, HU Lang, H. Lau, W. Schafer: Polypeptide syntheses. VIII. Formation of sulfur containing peptides by the intramolecular migration of aminoacyl groups. In: Liebigs Ann Chem. Volume 583, 1953, pp. 129-149.
- ^ PE Dawson, TW Muir, I. Clark-Lewis, SB Kent: Synthesis of proteins by native chemical ligation. In: Science. Volume 266, Number 5186, November 1994, ISSN 0036-8075 , pp. 776-779. PMID 7973629 .
- ↑ S. Kent: Total chemical synthesis of enzymes. In: Journal of peptide science: an official publication of the European Peptide Society. Volume 9, Number 9, September 2003, ISSN 1075-2617 , pp. 574-593, doi: 10.1002 / psc.475 . PMID 14552420 .