Neodymium (II) iodide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
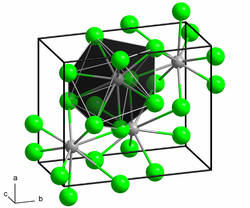
|
||||||||||||||||
| __ Nd 3+ __ I - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Neodymium (II) iodide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | NdI 2 | |||||||||||||||
| Brief description |
dark purple to black solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 398.05 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
5.85 g cm −3 |
|||||||||||||||
| Melting point |
562 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Neodymium (II) iodide is an inorganic chemical compound of neodymium from the group of iodides .
Extraction and presentation
Neodymium (II) iodide can be obtained by reducing neodymium (III) iodide with neodymium in a vacuum at 800 to 900 ° C.
It can also be represented by reacting neodymium with mercury (II) iodide .
Direct representation from iodine and neodymium is also possible.
The compound was first synthesized in 1961 by John D. Corbett.
properties
Neodymium (II) iodide is a dark purple to black solid. It is extremely hygroscopic and can only be stored and handled under carefully dried protective gas or in a high vacuum. In air, it changes into hydrates with moisture absorption , but these are unstable and more or less quickly transform into oxide iodides with the evolution of hydrogen. These processes take place much faster with water. The compound has a crystal structure of the strontium (II) bromide type. This transforms under pressure into the molybdenum disilicide structure typical of intermetallic compounds , which is already present in other rare earth diiodides ( e.g. for lanthanum diiodide ) under normal conditions. It forms complex compounds with tetrahydrofuran and other organic compounds.
use
Neodymium (II) iodide can be used as a reducing agent or catalyst in organic chemistry.
Individual evidence
- ↑ a b c d e f Georg Brauer , with the assistance of Marianne Baudler u. a. (Ed.): Handbook of Preparative Inorganic Chemistry . 3rd, revised edition. tape I . Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , pp. 1081 .
- ↑ AMERICAN ELEMENTS: Neodymium Iodide NdI2 , accessed May 2, 2014
- ↑ a b Datasheet Neodymium (II) iodide, anhydrous, powder, ≥99.9% trace metals basis from Sigma-Aldrich , accessed on May 2, 2014 ( PDF ).
- ^ Karl A. Jr. Gschneidner, Jean-Claude Bunzli, Vitalij K. Pecharsky: Handbook on the Physics and Chemistry of Rare Earths . Elsevier, 2009, ISBN 0-08-093257-6 , pp. 247 ( limited preview in Google Book search).
- ↑ Angelika Jungmann, R. Claessen, R. Zimmermann, G. e. Meng, P. Steiner, S. Hüfner, S. Tratzky, K. Stöwe, HP Beck: Photoemission of LaI 2 and CeI 2 . In: Zeitschrift für Physik B Condensed Matter. 97, 1995, pp. 25-34, doi : 10.1007 / BF01317584 .
- ↑ Ralf Alsfasser, Erwin Riedel: Modern Inorganic Chemistry . Walter de Gruyter, 2007, ISBN 3-11-019060-5 , p. 188 ( limited preview in Google Book search).
- ↑ Mikhail N. Bochkarev, Igor L. Fedushkin, Sebastian Dechert, Anatolii A. Fagin, Herbert Schumann: [NdI 2 (thf) 5 ], the first crystallographically characterized neodymium (II) complex. In: Angewandte Chemie. 113, 2001, pp. 3268-3270, doi : 10.1002 / 1521-3757 (20010903) 113: 17 <3268 :: AID-ANGE3268> 3.0.CO; 2-K .
- ↑ GV Khoroshen kov, AA Fagin, MN Bochkarev, S. Dechert, H. Schumann: Reactions of neodymium (II), dysprosium (II), and thulium (II) diiodides with cyclopentadiene In: Russian Chemical Bulletin. 52, pp. 1715-1719, doi : 10.1023 / A: 1026132017155 .
- ↑ Anatolii A. Fagin, Tatyana V. Balashova, Dmitrii M. Kusyaev, Tatyana I. Kulikova, Tatyana A. Glukhova, Natalya P. Makarenko, Yurii A. Kurskii, William J. Evans, Mikhail N. Bochkarev: Reactions of neodymium ( II) iodide with organohalides. In: Polyhedron. 25, 2006, pp. 1105-1110, doi : 10.1016 / j.poly.2005.08.050 .
- ↑ Fundamental Chemistry: Neodymium Based Ziegler Catalysts . Springer, 2006, ISBN 3-540-34809-3 , pp. 13 ( limited preview in Google Book search).
- ↑ Handbook on the Physics and Chemistry of Rare Earths . Elsevier, 2009, ISBN 0-08-093257-6 , pp. 261 ( limited preview in Google Book search).



