Nickel (II) chromate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
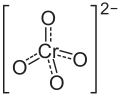
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Nickel (II) chromate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | NiCrO 4 | |||||||||||||||
| Brief description |
green odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 174.68 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.2 g cm −3 |
|||||||||||||||
| Vapor pressure |
1.5 hPa (20 ° C) |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Nickel chromate is an inorganic chemical compound of nickel from the group of chromates .
Extraction and presentation
Nickel chromate can be obtained by reacting nickel (II) carbonate with chromium trioxide at 260 ° C.
properties
As a hydrate, nickel chromate is a crystalline green, odorless solid that is soluble in water. It has an orthorhombic crystal structure with the space group Cmcm (space group no. 63) .
use
Nickel chromate is used as a catalyst .
Individual evidence
- ↑ a b c d e f Entry for CAS no. 14721-18-7 in the GESTIS substance database of the IFA , accessed on July 10, 2016(JavaScript required) .
- ↑ a b springer.com: NiCrO4 (Ni [CrO4]) Crystal Structure - SpringerMaterials ( Memento of the original from July 10, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed July 10, 2016
- ↑ a b Datasheet Nickel (II) chromate hydrate, 98% from AlfaAesar, accessed on July 10, 2016 ( PDF )(JavaScript required) .
- ↑ Entry on nickel chromate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Friend, J. Newton: A text-book of inorganic chemistry, Volume VI, Part III , Chromium and its Congeners, accessed July 10, 2016
- ↑ iaea.org: Table of Magnetic Structures Determined by Neutron Diffraction , A.Olos, F.Kajzar, M.Kuceb, accessed on July 10, 2016
- ↑ Dale L. Perry: Handbook of Inorganic Compounds . CRC Press, 1995, ISBN 978-0-8493-8671-8 , pp. 274 ( limited preview in Google Book search).


