Nickel formate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
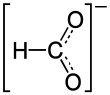 |
||||||||||
| General | ||||||||||
| Surname | Nickel formate | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 2 H 2 NiO 4 | |||||||||
| Brief description |
green odorless solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 148.73 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
2.154 g cm −3 |
|||||||||
| Melting point |
130-140 ° C |
|||||||||
| boiling point |
180–200 ° C (decomposition) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Nickel formate is the nickel salt of formic acid with the constitutional formula Ni (HCOO) 2 .
Extraction and presentation
Nickel formate can be obtained by reacting nickel (II) acetate or nickel (II) hydroxide with formic acid .
It is also possible to produce it by reacting sodium formate with nickel (II) sulfate .
properties
As a dihydrate, nickel formate is a green, odorless, non-flammable solid that is sparingly soluble in water. The compound has a monoclinic crystal structure . When carefully heated, the anhydrate forms at 130–140 ° C. When heated in a vacuum to 300 ° C, pure nickel is produced.
use
Nickel formate is used to make nickel and other nickel compounds such as nickel catalysts.
Individual evidence
- ↑ a b c d e f g h i Entry on nickel format in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b c d e Entry on nickel formate in the Hazardous Substances Data Bank , accessed June 29, 2015.
- ↑ a b G. WA Milne: Gardner's Commercially Important Chemicals Synonyms, Trade Names, and Properties . John Wiley & Sons, 2005, ISBN 0-471-73661-9 , pp. 738 ( limited preview in Google Book search).
- ↑ Entry on nickel diformate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A b John Kotz, Paul Treichel, John Townsend: Chemistry and Chemical Reactivity, Enhanced Edition . Cengage Learning, 2009, ISBN 978-0-495-39029-9 , pp. 335 ( limited preview in Google Book search).
- ↑ Jürgen Falbe, Manfred Regitz: RÖMPP Lexikon Chemie, 10th edition, 1996-1999 Volume 4: M - Pk . Georg Thieme Verlag, 2014, ISBN 3-13-200031-0 , p. 2238 ( limited preview in Google Book search).



