Niobium (V) ethoxide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
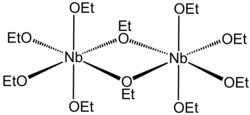
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Niobium (V) ethoxide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 25 NbO 5 | ||||||||||||||||||
| Brief description |
colorless to amber liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 318.21 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.258 g cm −3 |
||||||||||||||||||
| Melting point |
6 ° C |
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.5160 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Niobium (V) ethoxide is a chemical compound of niobium from the group of ethanolates .
Extraction and presentation
Niobium (V) ethoxide can be obtained by reacting niobium (V) chloride with ethanol in the presence of ammonia . The compound can also be obtained electrochemically directly from niobium .
properties
Niobium (V) ethoxide is a colorless to amber colored liquid that is soluble in organic solvents . Decomposition occurs in water.
use
Niobium (V) ethoxide is used for the production of niobium (V) oxide and mixed metal oxides. It is also used for sol-gel processing of materials containing niobium oxides and mixed metal oxides. It is also used to manufacture ferroelectric nanomaterials, which consist of oxides of potassium , silicon and niobium.
Individual evidence
- ↑ a b c d e f g h i j k l data sheet Niobium (V) ethoxide, 99.999% (metals basis), Ta <500ppm from AlfaAesar, accessed on March 29, 2020 ( PDF )(JavaScript required) .
- ^ A b c William M. Haynes: CRC Handbook of Chemistry and Physics . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 76 ( limited preview in Google Book search).
- ↑ Google Patents: US20020143200A1 - Process for preparing tantalum alkoxides and niobium alkoxides - Google Patents , accessed March 29, 2020
- ↑ DC Bradley, BN Chakravarti, W. Wardlaw: 460. Normal alkoxides of quinquevalent niobium. In: Journal of the Chemical Society. 1956, p. 2381, doi : 10.1039 / JR9560002381 .
- ↑ Ya-nan Cai, Sheng-hai Yang and a .: Electrochemical synthesis, characterization and thermal properties of niobium ethoxide. In: Journal of Central South University of Technology. 18, 2011, p. 73, doi : 10.1007 / s11771-011-0661-2 .

