Nitrification inhibitors
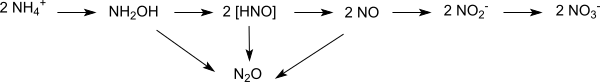
Nitrification inhibitors or nitrification inhibitors delay the bacterial oxidation of ammonium ions (NH 4 + ) , known as nitrification , by reducing the activity of Nitrosomonas bacteria for a certain period of time (four to ten weeks). Nitrosomonas metabolize ammonium ions to nitrite ions (NO 2 - ), which in turn are oxidized to nitrate (NO 3 - ) by Nitrobacter and Nitrosolobus . Recent research suggests that the role of ammonia-oxidizing archaea in nitrification has been significantly underestimated.
Ammonium and urea nitrogen fertilizers ( N fertilizers ) are added nitrification inhibitors in amounts of 0.8% 3,4-dimethylpyrazole phosphate (DMPP) to 10% dicyandiamide (DCD), based on total ammonium or carbamide nitrogen.
Mode of action
The effect of nitrification inhibitors is based on the reduction of nitrate losses through leaching and the formation of nitrous oxide (N 2 O) through denitrification in the upper soil layer by removing nitrogen from nitrogen fertilizers such as ammonium nitrate , ammonium sulfate , diammonium hydrogen phosphate ( ammonium phosphate ), ammonium sulfate nitrate or urea for longer Maintained ammonium form and so the nitrogen efficiency is significantly increased. By delaying the formation of nitrates from ammonium, nitrification inhibitors also avoid excessive amounts of nitrate in plants that serve as animal and human food.
However, the suppression of nitrification does not prevent mineral nitrogen, i.e. H. Nitrogen from mineral fertilizers is introduced into bodies of water through direct nitrogen application or water runoff.
Mechanism of action
In the first step of nitrification, ammonium ions are oxidized by nitrosomonas to nitrite ions (NO 2 - ). In the second step, nitrite to nitrate (NO 3 - ) is oxidized by Nitrobacter and Nitrosolobus.
Nitrification inhibitors inhibit the enzyme ammonium monooxygenase AMO in ammonia oxidizing bacteria of the genus Nitrosomonas and thus block the rate-determining step of the nitrification reaction.
In archaea of the phylum Thaumarchaeota, amo -like genes have also been found that code for the corresponding AMO subunits.
Denitrification processes running in parallel produce nitroxyl and nitrogen monoxide from the reactive intermediates formed as intermediates . These processes are also inhibited by nitrification inhibitors.
Chemical agents
In addition to acetylene and acetylene derivatives such as phenylacetylene and 2-ethynylpyridine, mainly substituted nitrogen heterocycles with two or three neighboring nitrogen atoms ( pyrazoles , 1,2,4-triazoles , pyridazines , benzotriazoles , indazoles ) were investigated for their suitability as nitrification inhibitors.
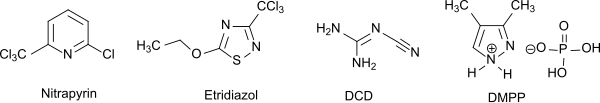
The most important nitrification inhibitors are nitrapyrin (mainly used in the USA), DCD (Europe, Asia, Oceania) and DMPP (Europe). There is also etridiazole .
Requirements for active ingredients
For use as nitrification inhibitors in horticulture and agriculture, the active ingredients must meet the following requirements:
- Stable during production, storage, transport and use
- Non-toxic to plants, animals and humans
- No degradation to toxic metabolites in the soil
- No negative effects on soil fertility
- Inexpensive with high efficiency
- Reduction of nitrate leaching without being washed out
- Reduction of nitrous oxide emissions ( greenhouse potential of N 2 O is 298)
- No negative effect on methane oxidation of the soil
This results in the following economic advantages when using nitrification inhibitors:
- Saving of application quantities of nitrogen fertilizer
- Reduction in the number of nitrogen fertilizations
- Increase in harvest volumes
- Higher crop yields with current amounts of nitrogen fertilizer applied
Nitrogen fertilization with nitrification inhibitors
Plants can only absorb nitrogen from the soil solution in the form of ammonium and nitrate ions through the roots. Ammonium ions adsorb on the surface of soil particles, while nitrate ions are free to move, are more quickly absorbed from the soil solution, but can also be easily washed out.
In water-saturated soils and at high temperatures, on the other hand, denitrification to nitrogen N 2 , nitrous oxide N 2 O (laughing gas) and nitrogen oxides NO x is favored.
Nitrogen losses through nitrate leaching, gas emissions through denitrification and ammonia volatilization and immobilization through firm binding to soil particles (humus) are ecologically and economically unfavorable and can be reduced through the use of special fertilizers (with delayed or controlled N release), optimized fertilization methods and nitrification inhibitors.
Therefore, the actual utilization of nitrogen from mineral fertilizers (this also includes urea according to the FAO conventions) is only 50–60% in the first year. It is estimated that cereal crops only take up 30 to 50% of the available fertilizer nitrogen.
The reduction in methane emissions - caused by the anaerobic degradation of cellulose in the soil - by stimulating methane oxidation is an additional benefit of some nitrification inhibitors.
A report by the “Scientific Advisory Council on Fertilization Issues” states that emissions of climate-relevant gases can be reduced by up to 50% for N 2 O and up to 35% for methane through the use of nitrification inhibitors .
The uptake of phosphorus and micronutrients can also be increased by nitrification inhibitors, which lengthen the ammonium phase of the nitrogen introduced into the soil.
rating
After hundreds of laboratory, greenhouse and field tests with different plants, soils, fertilization methods and techniques, weather conditions and climates, etc., a positive ecological and economic profile of the established nitrification inhibitors can be assumed. The required amounts of effort are very low at 0.5 to 1.5 kg / ha for DMPP.
The lower use of nitrogen fertilizers, the lower number of fertilization rounds, the reduced pollutant emissions in water and air, as well as the higher crop yields with unchanged nitrogen input, demonstrate the benefits of using nitrification inhibitors in agriculture and horticulture.
In addition, there are also publications that relativize and even question the effectiveness of nitrification inhibitors with regard to ammonia volatilization, nitrate leaching, crop yield and nitrogen uptake by plants.
literature
- ME Trenkel: Slow- and Controlled-Release Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture. 2nd ed., International Fertilizer Industry Association, Paris, October 2010, ISBN 978-2-9523139-7-1 ( online ).
- Johannes CG Ottow: Microbiology of Soils: Biodiversity, Ecophysiology and Metagenomics. Springer, Berlin / Heidelberg 2011, ISBN 978-3-642-00823-8 , doi: 10.1007 / 978-3-642-00824-5 .
Individual evidence
- ↑ R. Hatzenpichler: Diversity, Physiology, and Niche differentiation of Ammonia-Oxidizing Archaea . In: Appl. Environ. Microbiol. tape 78 , no. 21 , 2012, p. 7501-7510 , doi : 10.1128 / AEM.01960-12 .
- ↑ AH Treusch, S. Leininger, A. Kletzin, SC Schuster, H.-P. Klenk, C. Schleper: Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cyclin . In: Environm. Microbiol. tape 7 , no. 12 , 2005, p. 1985-1995 , doi : 10.1111 / j.1462-2920.2005.00906.x .
- ^ A. Weiske, G. Benckiser, T. Herbert, JCG Ottow: Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments . In: Biol. Fertil. Soils . tape 34 , 2001, p. 109-117 , doi : 10.1007 / s003740100386 .
- ↑ Statement of the Federal Government on the resolution of the Federal Council on the regulation on the amendment of fertilizer regulations Federal Council, printed matter 239/96 ( online ).
- ↑ W. Zerulla, T. Barth, J. Dressel, K. Erhardt, K. Horchler of Locquenghien, G. Pasda, M. Rädle, AH Know Meier: 3,4-Dimethylpyrazole phosphate (DMPP) - a new nitrification inhibitor for agriculture and horticulture . In: Biol. Fertil. Soils . tape 34 , 2001, p. 79-84 , doi : 10.1007 / s003740100380 .
- ↑ TH Misselbrook, LM Cardenas, V. Camp, RE Thorman, JR Williams, AJ Rollett, BJ Chambers: An assessment of nitrification inhibitors to reduce nitrous oxide emissions from UK agriculture . In: Environ. Res. Lett. tape 9 , 2014, p. 1–11 , doi : 10.1088 / 1748-9326 / 9/11/115006 .