Olympics
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
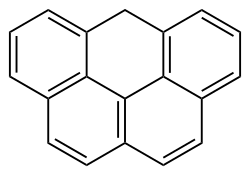
|
|||||||||||||
| General | |||||||||||||
| Surname | Olympics | ||||||||||||
| other names |
6 H -benzo [ cd ] pyrene |
||||||||||||
| Molecular formula | C 19 H 12 | ||||||||||||
| Brief description |
yellow needles |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 240.31 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
123-124 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Olympic is a hydrocarbon . The molecule consists of five rings, four of which are benzene rings, linked together similar to the shape of the Olympic rings.
The molecule was proposed in March 2010 by Graham Richards of the University of Oxford and Antony Williams in celebration of the 2012 London Olympics . It was first synthesized by Anish Mistry and David Fox from the University of Warwick in the UK. The relative energies of olympics and its isomers were initially predicted from quantum electronic structure calculations by Andrew Valentine and David Mazziotti of the University of Chicago .
Name- giving Olympic rings
Images of the molecule were made using scanning tunneling microscopy . More detailed images were made by IBM researchers in Zurich with the help of non-contact atomic force microscopy in 2012.
See also
Individual evidence
- ↑ a b Morgenthaler, J .; Ruechardt, C .: New Hydrogen Transfer Catalysts . In: Eur. J. Org. Chem. 1999, 2219-2230, doi : 10.1002 / (SICI) 1099-0690 (199909) 1999: 9 <2219 :: AID-EJOC2219> 3.0.CO; 2-D .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ AJ Williams: The Story of Olympicene from Concept to Completion . In: ChemConnector . Royal Society of Chemistry . May 27, 2012.
- ↑ A. Mistry: Dehydration of 3,4-dihydro-5 H -benzo [ cd ] pyrene-5-ol; 6 H -benzo [ cd ] pyrene . In: ChemSpider . Royal Society of Chemistry . May 31, 2012.
- ^ AJ Williams: Step by Step to the Synthesis of Olympicene . In: ChemConnector . Royal Society of Chemistry . March 14, 2012.
- ^ AJS Valentine, DA Mazziotti: Theoretical Prediction of the Structures and Energies of Olympicene and its Isomers . In: J. Phys. Chem. A . 117, No. 39, 2013, pp. 9746-9752. doi : 10.1021 / jp312384b .
- ↑ J. Palmer: 'Olympic rings' molecule olympicene in striking image . BBC News . May 28, 2012.

