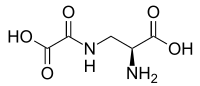
Oxalyldiaminopropionic acid
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Oxalyldiaminopropionic acid | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 5 H 8 N 2 O 5 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 176.13 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
206 ° C (disintegration of the monohydrate) |
||||||||||||
| pK s value |
1.95 |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Oxalyldiaminopropionic acid is a non-proteinogenic amino acid found in the common pea ( Lathyrus sativus ). The substance is neurotoxic and is believed to cause lathyrism . It leads to cramps and spastic paralysis . This is probably a consequence of the similarity with L -glutamate .
biosynthesis
Oxalyldiaminopropionic acid is synthesized in the plant from L- asparagine .
Crystallization
A metastable monohydrate and a stable anhydrate exist as crystal forms .
Individual evidence
- ↑ Haskell, BE; Bowlus, SB: in J. Org. Chem. 41 : 159-160 (1976).
- ↑ a b c d Paul O'Brien, Peter B. Nunn: Dimorphism of β-n-oxalyl-l- α, β- diaminopropionic acid . In: Phytochemistry . tape 21 , no. January 8 , 1982, ISSN 0031-9422 , pp. 2001-2004 , doi : 10.1016 / 0031-9422 (82) 83031-8 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on oxalyldiaminopropionic acid in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ BA Warren, SA Patel, PB Nunn, RJ Bridges: The Lathyrus excitotoxin β-N-oxalyl-l- α, β- diaminopropionic acid is a substrate of the L-cystine / L-glutamate exchanger system x c - . In: Toxicology and Applied Pharmacology . tape 200 , no. 2 , 2004, p. 83-92 , doi : 10.1016 / j.taap.2004.04.001 .
- ^ PM Dey, JB Harborne, Plant Biochemistry . Elsevier, 1997. ISBN 978-0-12-214674-9 . P. 442.