Tolualdehyde
| Tolualdehyde | ||||||
| Surname | 2-tolualdehyde | 3-tolualdehyde | 4-tolualdehyde | |||
| other names |
o -Tolualdehyde, 2-methylbenzaldehyde |
m -Tolualdehyde, 3-methylbenzaldehyde |
p -Tolualdehyde, 4-methylbenzaldehyde |
|||
| Structural formula |

|
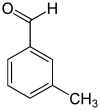
|

|
|||
| CAS number | 529-20-4 | 620-23-5 | 104-87-0 | |||
| PubChem | 10722 | 12105 | 7725 | |||
| Molecular formula | C 8 H 8 O | |||||
| Molar mass | 120.15 g mol −1 | |||||
| Physical state | liquid | |||||
| Melting point | −35 ° C | - | −6 ° C | |||
| boiling point | 198-200 ° C | 199 ° C | 204 ° C | |||
|
GHS labeling |
|
|
|
|||
| H and P phrases | 302-315-319-335 | no H-phrases | 302 | |||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||
|
261-280-302 + 352-305 + 351 + 338 304 + 340-405-501 |
no P-phrases | 262 | ||||
In chemistry, tolualdehydes form a group of substances that are derived from both benzaldehyde and toluene . The structure consists of a benzene ring with attached aldehyde (-CHO) and methyl groups (-CH 3 ) as substituents . Their different arrangement results in three constitutional isomers with the empirical formula C 8 H 8 O. They are mainly to be regarded as methyl-substituted benzaldehydes.
presentation
4-Tolualdehyde 2 can be prepared by Friedel-Crafts acylation of toluene 1 with carbon monoxide and hydrogen chloride under Gattermann-Koch conditions .
See also
Individual evidence
- ↑ a b c Entry for CAS no. 529-20-4 in the GESTIS substance database of the IFA , accessed on March 25, 2017(JavaScript required) .
- ↑ a b Entry for CAS no. 620-23-5 in the GESTIS substance database of the IFA , accessed on March 25, 2017(JavaScript required) .
- ↑ a b c Entry for CAS no. 104-87-0 in the GESTIS substance database of the IFA , accessed on March 25, 2017(JavaScript required) .
- ^ GH Coleman, David Craig: p-Tolualdehyde In: Organic Syntheses . 12, 1932, p. 80, doi : 10.15227 / orgsyn.012.0080 ; Coll. Vol. 2, 1943, p. 583 ( PDF ).

