Palladium (II) bromide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
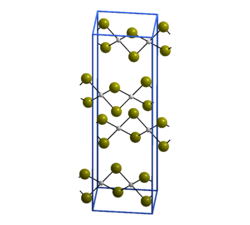
| Crystal structure of palladium (II) bromide __ Pd 2+ __ Br - |
||||||||||||||||
| General | ||||||||||||||||
| Surname | Palladium (II) bromide | |||||||||||||||
| other names |
Palladium dibromide |
|||||||||||||||
| Ratio formula | PdBr 2 | |||||||||||||||
| Brief description |
black solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 266.22 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
5.35 g cm −3 |
|||||||||||||||
| Melting point |
310 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Palladium (II) bromide is an inorganic chemical compound of palladium from the group of bromides .
Extraction and presentation
Palladium (II) bromide can be obtained by reacting palladium with a hydrogen bromide - bromine solution. It can also be obtained directly from the elements.
properties
Palladium (II) bromide is a brown-black solid that is in the form of needle-shaped crystals. It is insoluble in water and organic solvents, but soluble in hydrohalic acids. It crystallizes monoclinically in the space group P 2 1 / c (space group no. 14) with the lattice parameters a = 659 pm, b = 396 pm, c = 2522 pm and β = 92.6 ° as well as four formula units per unit cell . In the crystal, each palladium atom is surrounded by four bromine atoms in a square-planar manner, each bromine atom binds to another palladium atom, so that infinite zigzag chains are created.
use
Palladium (II) bromide can be used as a catalyst in organic chemistry, although it is sometimes more active than palladium (II) chloride .
Individual evidence
- ↑ a b c d e data sheet Palladium (II) bromide, Premion®, 99.998% (metals basis), Pd 39.5% min from AlfaAesar, accessed on August 30, 2013 ( PDF )(JavaScript required) .
- ↑ a b c d Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer, 1998, ISBN 3-642-58842-5 , pp. 668 ( limited preview in Google Book search).
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1730.
- ↑ Catherine E. Housecroft: Inorganic Chemistry . Pearson Education, 2005, ISBN 0-13-039913-2 , pp. 686 ( limited preview in Google Book Search).
- ^ Jiro Tsuji : Palladium Reagents and Catalysts: New Perspectives for the 21st Century . John Wiley & Sons, 2006, ISBN 0-470-02119-5 , pp. 344 ( limited preview in Google Book search).
