Pantothenic acid
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
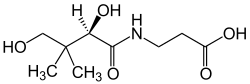
|
|||||||||
| Structural formula of the ( R ) -enantiomer | |||||||||
| General | |||||||||
| Common name | Vitamin B 5 | ||||||||
| other names |
|
||||||||
| Molecular formula | C 9 H 17 NO 5 | ||||||||
| CAS number |
|
||||||||
| PubChem | 988 | ||||||||
| ATC code | |||||||||
| Brief description |
|
||||||||
| Occurrence | in all animal and vegetable foods | ||||||||
| physiology | |||||||||
| function | Part of coenzyme A and the acyl carrier protein of fatty acid synthase, participates in wound healing | ||||||||
| Daily need | 6 mg | ||||||||
| Consequences in case of deficiency | Fatigue, insomnia, depression, numb or sore muscles, anemia, immune deficiencies, stomach pain | ||||||||
| Overdose | 10 g | ||||||||
| properties | |||||||||
| Molar mass | 219.24 g mol −1 | ||||||||
| Physical state | liquid | ||||||||
| Melting point |
|
||||||||
| solubility |
|
||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Pantothenic acid , also known as vitamin B 5 , is a water-soluble vitamin from the series of B vitamins and a derivative of β-alanine .
function
Pantothenic acid is necessary for the development of coenzyme A , which catalyzes the transfer of acyl groups in the metabolism (e.g. in the citric acid cycle or fatty acid oxidation / biosynthesis as acetyl-CoA , succinyl-CoA , malonyl-CoA ). It is involved in the build- up and breakdown of carbohydrates , fats and the synthesis of cholesterol , which is needed for the formation of steroid hormones .
biosynthesis
In vertebrates (including humans ), pantothenic acid can be formed from the provitamin dexpanthenol .
Pantothenic acid is formed in bacteria and archaea , among other things, during the condensation reaction between pantoic acid and β-alanine . Pantothenic acid is chiral , so there are two enantiomers of pantothenic acid, ( R ) - N - (2,4-dihydroxy-3,3-dimethyl-1-oxobutyl) - β- alanine and ( S ) - N - (2,4 dihydroxy-3,3-dimethyl-1-oxobutyl) - β -alanine, and the racemate [1: 1 mixture of the ( R ) - and ( S ) enantiomer]. Unless expressly stated otherwise in this article or in the specialist literature, all statements about pantothenic acid relate to the naturally occurring pure ( R ) -enantiomer.
transport
In humans, pantothenic acid is absorbed by the transport protein SMVT ( sodium multi-vitamin transporter ).
Excess pantothenic acid is quickly released into the urine.
Occurrence
Pantothenic acid occurs in particular in offal, whole grain products, avocado, eggs, nuts (especially pine nuts), rice, fruit, vegetables, milk and brewer's yeast.
requirement
The 6 mg / day requirement is usually met through a normal diet. An undersupply is very rare, but can occur, for example, in connection with intestinal diseases, alcohol abuse or chronic inflammation.
Deficiency symptoms
An isolated deficiency of pantothenic acid as hypovitaminosis is rare; rather, the body usually lacks other B group vitamins. Deficiency can lead to fatigue, insomnia, depression, numb or sore muscles, anemia , immune deficiencies, and stomach pain.
The so-called Burning Feet Syndrome ( burning feet ) occurs after a three to four month pantothenic acid deficiency. The symptoms are initially tingling and numbness in the toes, followed by burning and stinging in the feet. These complaints are accompanied by psychological and neurological symptoms such as muscle tension or nerve irritation. The syndrome became known during the Second World War among prisoners of war in Burma, the Philippines and Japan who suffered from pantothenic acid deficiency.
Consequences of an overdose
When taking well over 10 g pantothenic acid per day for months, it can lead to slight intestinal disorders as hypervitaminosis , and overdosing by more than a thousand times the maximum recommendation to gastrointestinal disorders or diarrhea .
history
Pantothenic acid was discovered in 1931 by the American nutritional biologist and biochemist Roger John Williams as a substance that promotes the growth of yeast.
use
In the form of the sodium salt or calcium salt or of ( R ) -panthenol, pantothenic acid is used medicinally for wound healing as well as in hair treatment products and feed additives. It is used topically in the form of dexpanthenol for skin injuries, acne and hair loss. The effect is controversial. It is also contained in so-called energy drinks .
Web links
- D'Eustachio / reactome.org: Pantothenate transport across the plasma membrane (Engl.)
- Swiss Forum For Sport Nutrition: Information sheet on pantothenic acid ( Memento from March 17, 2007 in the Internet Archive ) (PDF; 20 kB)
Individual evidence
- ↑ a b c d e Entry on calcium D-pantothenate in the GESTIS substance database of the IFA , accessed on June 25, 2017(JavaScript required) .
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer : Biochemistry. 6 edition, Spektrum Akademischer Verlag, Heidelberg 2007. ISBN 978-3-8274-1800-5 . (free full text access) .
- ↑ James E. Darnell , Harvey Lodish, David Baltimore : Molecular Cell Biology . de Gruyter, Berlin et al. 2001, ISBN 3-11-011934-X (4th edition. Harvey Lodish: Molecular Cell Biology. Spectrum Academic Publishing House, Heidelberg et al. 2001, ISBN 3-8274-1077-0 ). (free full text access) .
- ↑ F. Rébeillé, S. Ravanel, A. Marquet, RR Mendel, ME Webb, AG Smith, MJ Warren: Roles of vitamins B5, B8, B9, B12 and molybdenum cofactor at cellular and organismal levels. In: Natural Product Reports . Volume 24, Number 5, October 2007, pp. 949-962, doi : 10.1039 / b703104c . PMID 17898891 .
- ↑ SR Chirapu, CJ Rotter, EL Miller, MV Varma, RL Dow, MG Finn: High specificity in response of the sodium-dependent multivitamin transporter to derivatives of pantothenic acid. In: Current Topics in Medicinal Chemistry . Volume 13, Number 7, 2013, pp. 837-842, PMID 23578027 .
- ^ A b Gabriel a Camporeale, Rocio Rodriguez-Melendez, Janos Zempleni: Pantothenic acid and biotin. In: Ira Wolinsky, Judy A. Driskell (Eds.): Sports Nutrition: Vitamins and Trace Elements. CRC Press, Chapter 9, Oct 31, 2005, p. 312.
- ^ A b J. W. Miller, LM Rogers, RB Rucker: Panthotenic acid. In: BA Bowman, RM Russell (eds.): Present Knowledge in Nutrition. ILSI Press, Washington DC 2001, p. 253.
- ↑ RÖMPP Lexicon Food Chemistry, 2nd edition, 2006 . Georg Thieme Verlag, 2014, ISBN 978-3-13-179282-2 , p. 838 ( limited preview in Google Book search).
- ↑ Short textbook pharmacology and toxicology . Georg Thieme Verlag, 2013, ISBN 978-3-13-168033-4 ( limited preview in Google book search).
- ↑ Foodwatch: fact sheet Energy Shots and Energy Drinks from June 28, 2013, accessed on June 7, 2017.