Protriptyline
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
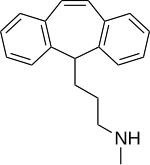
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Protriptyline | ||||||||||||
| other names |
3- (5 H -dibenzo [ a , d ] [7] annulen-5-yl) - N -methylpropan-1-amine |
||||||||||||
| Molecular formula | C 19 H 21 N | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 263.37 g mol −1
|
||||||||||||
| Melting point |
168 ° C (hydrochloride) |
||||||||||||
| pK s value |
8.2 |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Protriptyline ( trade names : Vivactil ® , Concordin ® ) is a drug from the group of tricyclic antidepressants . As strongly as desipramine, it inhibits the reuptake of the neurotransmitter norepinephrine , without having any sedative properties worth mentioning . Protriptyline also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ). The active ingredient was patented by Merck & Co. in 1962 . The hydrochloride is used .
Clinical information
Application areas (indications)
Due to the above-mentioned mechanism of action, the drug is particularly suitable for the treatment of depression with pronounced lack of drive. Treatment successes can also be achieved with ADHD , narcolepsy and chronic pain syndrome .
Adverse effects (side effects)
With protriptyline are u. a. determine the following undesirable effects:
- moderate to severe: effects on cardiac function
- moderate: anticholinergic effects and epileptic seizures
- low: low blood pressure ( hypotension ) and weight gain
- minimal: sedation
dosage
The usual daily dose is between 15 and 40 mg, the extreme doses are between 10 and 60 mg per day.
Pharmacological properties
The plasma elimination half-life of the drug is relatively long at 54 to 198 hours.
Individual evidence
- ↑ a b c Entry on protriptyline. In: Römpp Online . Georg Thieme Verlag, accessed on June 27, 2019.
- ↑ a b Data sheet Protriptyline hydrochloride from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ↑ a b Entry on protriptyline in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P: Identification of novel functional inhibitors of acid sphingomyelinase . In: PLoS ONE . 6, No. 8, 2011, p. E23852. doi : 10.1371 / journal.pone.0023852 .
- ^ A b Goodman & Gilman's: The pharmacological basis of therapeutics. 9th ed. McGraw-Hill, New York et al. a. 1996. p. 434.
- ↑ Hermann J. Roth u. Helmut Fenner: Drugs. Thieme, Stuttgart a. New York 1988. p. 238.