Desipramine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
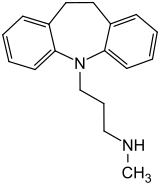
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Desipramine | |||||||||||||||||||||
| other names |
10,11-dihydro-5- [3- (methylamino) propyl] -5 H -dibenzo [ b, f ] azepine |
|||||||||||||||||||||
| Molecular formula | C 18 H 22 N 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 266.38 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Desipramine is a drug that belongs to the class of tricyclic antidepressants . It has a strong drive-enhancing effect (thymoleptic) and, after some time, mood-enhancing (thymoleptic) and is indicated for the treatment of depressive illnesses. Desipramin was launched on the German market in 1965 under the Pertofran brand . Today, finished medicinal products containing desipramine are out of distribution in most countries.
Presentation and extraction
The synthesis is based on 10,11-dihydro-5 H -dibenz [ b , f ] azepine, the target compound being obtained by a two-stage alkylation first with 1-bromo-3-chloropropane and then with methylamine .
pharmacology
Mechanism of action
Desipramine is the active metabolite of imipramine . It works in the central nervous system and inhibits the reuptake of serotonin , noradrenaline and other neurotransmitters from the synaptic gap into the presynaptic vesicles , thereby increasing their concentration and alleviating depressive symptoms. The sedative active component is weak. Furthermore, desipramine suppresses the perception of pain by acting on the nociceptors (antinociceptive effect). Desipramine also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
Side effects
The most common undesirable effects of desipramine, such as urination disorders ( micturition disorders ), inner restlessness and a feeling of thirst, as well as occasional undesirable effects such as confusion, intestinal paralysis or obstruction ( paralytic ileus ) and urinary retention, result from its anticholinergic effect.
Desipramine can prolong the QT time of the electrocardiogram (EKG) up to a dangerous arrhythmia, the torsade de pointes . A recent heart attack , severe AV blocks and other cardiac arrhythmias are therefore contraindications for treatment with desipramine. In December 2009, the US Food and Drug Administration (FDA) ordered the inclusion of a warning in the product information for Norpramin regarding particularly careful treatment of patients with a family history of incidents such as sudden cardiac death, cardiac arrhythmias or cardiac conduction disorders.
For some tricyclic antidepressants including desipramine, an increase in the risk of breast cancer is being discussed, although a number of reviews and meta-analyzes have not been able to confirm this hypothesis.
Interactions
The effects of desipramine and alcohol as well as other centrally active drugs ( barbiturates , pain relievers, antihistamines , antipsychotics ) can mutually reinforce one another. Desipramine also interacts with substances that attack the same receptors ( anticholinergics , serotonin reuptake inhibitors (SRI), α- sympathomimetics ) that prolong the QT time in the ECG (for example certain antiarrhythmics , antibiotics , anti- malarial drugs, neuroleptics ) or the affect the metabolism of desipramine.
Simultaneous treatment with MAO inhibitors is contraindicated , as serious adverse effects can occur. A therapy-free time (washout phase) must be observed between treatments with desipramine and MAO inhibitors.
Pharmacokinetics
Desipramine is well absorbed from the intestine, but due to its high first-pass effect it has a reduced bioavailability , which can also fluctuate greatly. The plasma half-life is 15 to 25 hours. Desipramine crosses the blood-brain and placental barrier and is excreted in breast milk. After biotransformation in the liver, desipramine is excreted via the kidneys (renally).
Individual evidence
- ↑ a b Data sheet Desipramine hydrochloride from Sigma-Aldrich , accessed on March 24, 2011 ( PDF ).
- ↑ a b c d e f g h i A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme-Verlag Stuttgart 2000, ISBN 978-1-58890-031-9 .
- ^ Gustav Ehrhart / Heinrich Ruschig : Medicines , 1968
- ↑ Psychiatric Drugs Timeline
- ↑ List of pharmaceutical substances in the ABDA database (May 2010).
- ↑ a b c Petylyl: Information for professionals. As of May 2008.
- ↑ J. Kornhuber, M. Muehlbacher, S. Trapp, S. Pechmann, A. Friedl, M. Reichel, C. Mühle, L. Terfloth, T. Groemer, G. Spitzer, K. Liedl, E. Gulbins, P Tripal: Identification of Novel Functional Inhibitors of Acid Sphingomyelinase . In: PLoS ONE . tape 6 , no. 8 , 2011, p. e23852 , doi : 10.1371 / journal.pone.0023852 .
- ↑ Norpramin (desipramine hydrochloride) - Dear Healthcare Professional Letter .
- ↑ CR Sharpe, JP Collet, E. Belzile, JA Hanley, JF Boivin: The effects of tricyclic antidepressants on breast cancer risk . (PDF; 94 kB). In: British Journal of Cancer . Volume 86, 2002, pp. 92-97. PMID 11857018 .
- ↑ DA Lawlor, P. Jüni, S. Ebrahim, M. Egger: Systematic review of the epidemiologic and trial evidence of an association between antidepressant medication and breast cancer. In: J Clin Epidemiol. 56 (2), Feb 2003, pp. 155-163. PMID 12654410 .
- ↑ S. Bahl, M. Cotterchio, N. Kreiger: Use of antidepressant medications and the possible association with breast cancer risk. A review. In: Psychother Psychosom. 72 (4), Jul-Aug 2003, pp. 185-194. PMID 12792123 .
- ^ PF Coogan: Review of the epidemiological literature on antidepressant use and breast cancer risk. In: Expert Rev Neurother . 6 (9), Sep 2006, pp. 1363-1374. PMID 17009923 .
- ↑ CS Eom, SM Park, KH Cho: Use of antidepressants and the risk of breast cancer: a meta-analysis In: Breast Cancer Res Treat . 136, 2012, pp. 635-645. PMID 23139055 .
