Antibiotic-associated colitis
| Classification according to ICD-10 | |
|---|---|
| A04.7 | Enterocolitis due to Clostridium difficile - pseudomembranous colitis |
| ICD-10 online (WHO version 2019) | |
An antibiotic-associated colitis and pseudomembranous colitis occurs when the intestinal flora (usually iatrogenic reasons) by antibiotics is so damaged that in this way in particular the bacterium Clostridioides difficile heavily can multiply. The toxins excreted by the clostridia cause fever, abdominal pain, diarrhea, and fluid loss.
Occurrence
Clostridioides difficile is a gram-positive , anaerobic bacterium and not a component of the physiological intestinal flora, although it can be detected in adults in 3–7% of cases and in infants in over 50% of cases without them becoming ill. Hospitalized patients have a 40% chance of shedding Clostridioides difficile.
Any antibiotic can cause antibiotic-associated colitis for up to four weeks after it is stopped, even those that are usually used to treat it.
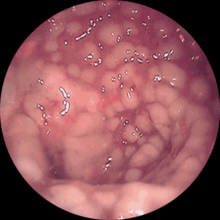
The toxins excreted by the clostridia ( large clostridial cytotoxins : toxin A and toxin B) can lead to diarrhea with a sometimes very severe course, which can include life-threatening fluid loss due to (bloody) diarrhea. The poison of the Clostridioides difficile destroys the mucous membrane layers and it comes to " volcano-like " fibrin exudation , which is described in the colonoscopy as "cat's head".
In 2002, the NAP1 strain (North American Pulsed field type 1) was described in North America, which can produce significantly more toxins and is therefore considered to be more virulent.
Symptoms
Diarrhea and abdominal cramps are the main symptoms of the disease, but they can also have many other causes. Only around 10–20% of all patients with suspected C. difficile are also positive in the laboratory test. In many cases, both patients and doctors described the chair as characteristically malodorous.
Risk factors
Since Clostridioides difficile is largely destroyed in the acidic environment of the stomach, proton pump inhibitors represent a risk factor for antibiotic-associated diarrhea .
In addition, different antibiotics favor colonization with clostridia more than others; Particularly noteworthy are lincosamides , cephalosporins and quinolone antibiotics . Antibiotics disrupt the physiological intestinal flora, which functions as “placeholders” in a healthy environment. In addition, they cause shifts in the bile acid balance, so that the reproduction of vegetative forms of C. difficile is no longer sufficiently inhibited.
diagnosis

Fever and leukocytosis are indicative of colitis caused by C. difficile . This can reach very high values (> 30,000 / mL). According to the US-American guidelines of the SHEA , the disease is classified as severe from 15,000 / mL or if the kidneys are damaged ( creatinine increase by 1.5 times). The leukocyte count correlates with the risk of death.
In the ultrasound and in the computed tomography one can see the elongated intestinal wall thickening of the large intestine. Endoscopically you can see greenish fibrin coatings in the large intestine, some of which are spotty, some of which are flat.
The final diagnosis is made through various tests of the stool for the presence of the pathogen or its toxins. The cytotoxin assay is considered the gold standard. Fibroblasts are contaminated with samples of the stool. In the presence of clostridial toxins, characteristic changes are visible under the microscope. These changes are in turn reversed by antitoxin to confirm the diagnosis. However, the test is technically complex and takes two to three days. A toxin-producing culture is also possible. Other bacteria are destroyed by heat or alcohol and the cell culture is checked for the presence of toxins. The test takes around 3–5 days. Both tests are very sensitive and relatively specific .
The most common test, however, is an enzyme immunoassay against the toxins themselves or an enzyme ( glutamate dehydrogenase ) from the bacteria. Both tests are less sensitive than the previous methods. However, they are technically simple, inexpensive and can be carried out within hours. The test for the bacterial enzyme only shows the presence of the bacteria, not their toxin production. As a result, it makes sense to combine both tests. A PCR , which detects the bacterium's DNA , is just as quick, but more expensive .
Differential diagnosis
Pseudomembranous colitis must be differentiated from simple, antibiotic-associated diarrhea , which is not triggered by an inflammation of the intestine.
A rarer form of antibiotic-associated colitis is antibiotic-associated hemorrhagic colitis caused by klebsiae . It often affects young, otherwise healthy patients and is associated with abdominal cramps and bloody diarrhea.
therapy
The first measure of therapy is, if possible, stopping the triggering antibiotic, as well as supporting the water and electrolyte balance. Metronidazole is considered the standard agent in clinical practice . In general, vancomycin is reserved as a reserve drug (in the case of metronidazole intolerance or allergy, pregnancy, children under ten years of age or metronidazole resistance) or in addition to metronidazole for more severe courses (with more than four bowel movements per day, poor general well-being, incipient fever and signs of inflammation). If therapy is resistant, a trial with bacitracin, fusidic acid or teicoplanin is recommended, although these alternatives should result in a higher recurrence rate than with metronidazole and vancomycin. A systematic Cochrane review, on the other hand, came to the conclusion that vancomycin and teicoplanin are most effective in symptomatic healing of the patient. However, teicoplanin is very expensive and little available. Teicoplanin offers a higher likelihood that the germ will no longer be detectable in a patient's intestine, and thus a way of limiting nosocomial infections in healthcare facilities.
Around a fifth of patients suffer a relapse, usually after successful drug treatment. This is caused in half of the cases by repopulation with Clostridioides difficile, in the other half by incomplete elimination of the germ. In the event of a first relapse, repeat therapy with metronidazole or vancomycin in the same dosage and duration is sought. In the event of a second relapse, a longer dose over 7 weeks has proven to be more effective. In May 2011, the US Food and Drug Administration approved fidaxomicin, a macrocycline antibiotic that was specially developed for use against Clostridioides difficile. Broad clinical evaluation of the active ingredient is still pending. The European Medicines Agency granted approval for the indication of clostridial-associated enteritis in September 2011.
In many experiments, the so-called fecal bacteriotherapy (colloquially “ stool transplantation ”) has proven to be promising. The approach is based on the assumption that damaged intestinal flora - which often happens after antibiotic treatment - strongly encourages relapses, since Clostridioides difficile germs can multiply unhindered thanks to the lack of competition. In this uncomplicated treatment, the stool of a healthy donor is pureed together with physiological saline solution and given into the patient's colon via an enema . The stool can also be administered through a nasoduodenal tube or with capsules. According to a recent meta-study, 92% of the patients were permanently cured. In 2014, similarly good results were achieved in a first pilot study with orally taken active ingredient encapsulated in pill form.
In rare cases, surgical therapy may be necessary. Total removal of the large intestine is considered superior to partial removal.
Hygiene measures
In order to prevent the spread of infection, certain hygiene measures must be strictly observed when dealing with the sick. This includes basic hygiene , whereby hand washing is of particular importance: In the event of contact with certain spore-forming pathogens such as Clostridioides difficile, the only way to reduce any adhering spores is by washing your hands, as they are not inactivated by the usual alcohol-based skin disinfectants.
In addition, the Commission for Hospital Hygiene and Infection Prevention (KRINKO) recommends, in addition to barrier measures, in case of suspected or diagnosed Clostridioides difficile infection (CDI), to accommodate the symptomatic patient in a single room with their own wet room and to maintain this isolation for at least 48 hours after the symptoms of diarrhea have ceased .
Complications
As a result, as with any severe diarrheal illness, a lack of fluids ( dehydration ) occurs. As a further complication to toxic megacolon , a perforation of the colon, a through migration peritonitis and septic shock may occur.
This course of the disease is known as fulminant colitis. Around four percent of patients have a brilliant course. Three factors could be identified as independent predictors of a fatal outcome: age over 70 years, excessive increase or decrease in white blood cells, and failure of the cardiovascular system with the need for medical circulatory support or intubation . A strain named NAP1 / 027, which was first isolated in an epidemic in Québec in 2002 and showed toxin overproduction in vitro , has been linked to more serious disease courses .
Research history
The first association with the antibiotic clindamycin and tissue description of the disease occurred during the 1970s. The disease was first attributed to Clostridium difficile in 1977 by a research group led by John Bartlett .
Economic importance
Cases of antibiotic-associated colitis require numerous, cost-intensive measures in hospitals and care facilities. In particular, an epidemic-like spread among hospitalized patients can result in significant operational disruptions up to and including the closure of entire departments. Estimates made in the middle of the last decade assume costs of around 3 billion euros per year in the European Union and 1.1 billion US dollars in the USA.
See also
literature
- Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , pp. 191-193.
Individual evidence
- ↑ a b Gerd Herold and colleagues: Internal medicine. Cologne 2011, ISBN 978-3-9814660-2-7 , pp. 842f.
- ↑ a b c d e T. Schneider, T. Eckmanns, R. Ignatius, K. Weist, O. Liesenfeld: Clostridium difficile-associated diarrhea. In: Deutsches Ärzteblatt. Vol. 104, issue 22, June 1, 2007, pp. 1588–1594.
- ↑ a b c d e J. G. Bartlett: Clostridium difficile: progress and challenges. In: Ann NY Acad Sci. 2010 Dec; 1213, pp. 62-69, PMID 21175676
- ↑ M. Aseeri, T. Schroeder, J. Kramer, R. Zackula: Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. In: Am J Gastroenterol . 2008 Sep; 103 (9), pp. 2308-2313, PMID 18702653 .
- ↑ KRINKO recommendation: hygiene measures for Clostridioides difficile infection (CDI). 2019, p. 907. Retrieved September 26, 2019.
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 191 f.
- ↑ Marianne Abele-Horn (2009), p. 192.
- ^ R. Nelson: Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. In: Cochrane Database Syst Rev. 2007 Jul 18; (3), S. CD004610. PMID 17636768
- ↑ FDA publication, available as html , last accessed on May 30, 2011.
- ↑ EMA press release of September 24, 2011 available online as pdf ; last accessed on September 24, 2011.
- ↑ Gough, Shaikh & Manges: Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium difficile Infection. In: Clinical Infectious Diseases . (2011); vol. 53, pp. 994-1002. (Abstract)
- ↑ Youngster, Russell, Pindar, Ziv-Baran, Sauk, Hohmann: Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. In: JAMA Network . October 11, 2014.
- ↑ KRINKO recommendation: hygiene measures for Clostridioides difficile infection (CDI). 2019, p. 912. Retrieved September 26, 2019.
- ↑ KRINKO recommendation: hygiene measures for Clostridioides difficile infection (CDI). 2019, pp. 912 and 916. Retrieved September 26, 2019.
- ^ Gerd Herold and colleagues: Internal medicine. Cologne 2013, p. 867 f.
- ↑ EA Sail Hamer, K. Carson, Y. Chang, N. Zacharias include: Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. In: Arch Surg . 2009 May; 144 (5), pp. 433-439; discussion 439-440. PMID 19451485 .
- ↑ M. Warny, J. Pepin, A. Fang, G. Killgore, A. Thompson et al: Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. In: Lancet. 2005 Sep 24-30; 366 (9491), pp. 1079-1084. PMID 16182895 .