PyBOP
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
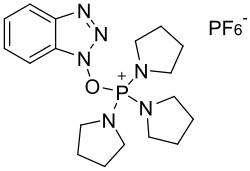
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | PyBOP | ||||||||||||||||||
| other names |
Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate |
||||||||||||||||||
| Molecular formula | C 18 H 28 F 6 N 6 OP 2 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 520.39 g mol −1 | ||||||||||||||||||
| Physical state |
solid, crystalline |
||||||||||||||||||
| Melting point |
156-157 ° C |
||||||||||||||||||
| solubility |
soluble in dichloromethane |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
PyBOP (benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate) is one of the organic phosphonium salts and is used as a coupling reagent in peptide synthesis . This compound, introduced by the group around Castro in 1990, represents a further development of the phosphonium salt BOP , which, unlike the latter, does not release highly toxic HMPT after coupling.
presentation
The original synthesis of PyBOP starts from tripyrrolidinophosphine oxide, which is converted into the chlorophosphonium salt using phosgene . This is followed by reaction with the triethylammonium salt of 1-hydroxybenzotriazole (HOBt), which is accessible from HOBt and triethylamine (Et 3 N), whereby a chloride is substituted for OBt. The last step is the exchange of the chloride anion for a weakly coordinating hexafluorophosphate anion . Instead of gaseous phosgene, solid triphosgene or phosphoryl chloride can also be used.
The structurally related BOP can also be represented in this way. In the BOP, only the pyrrolidino or pyrrolidin-1-id are substituted by dimethylamide groups.
properties
The connection is thermally unstable. A DSC measurement from 121 ° C shows a very strong exothermic decomposition reaction with an exothermicity of −1020 kJ kg −1 or −530.8 kJ mol −1 .
use
Coupling reagent
PyBOP is used as a coupling reagent in peptide synthesis . The activation is realized via the phosphonium salt 2 or the OBt active ester 3 . The latter is known to suppress racemization , but also reacts more slowly with an amine 4 to form the peptide or, in general, the amide . One of the driving forces behind the reaction is the formation of stable phosphoric acid triamide 5 .
In this scheme, B stands for base. Mostly PyBOP is used together with DIPEA .
Other reactions
PyBOP means also can Thiolsäureester 6 combine with amines, but in addition to the amide 8 the thionamide 7 is formed, thereby providing easy access to this class of compounds. The typical synthesis of thionamides involves the reaction of an amide with Lawesson's reagent . The reason for the O / S selectivity shown here is the stronger bond between phosphorus and oxygen.
Nitriles can be prepared by reacting amides with PyBOP / DIPEA . Here, too, the oxygen of the carbonyl group nucleophilically attacks the phosphorus atom of the PyBOP. In the presence of a base, the hexafluorophosphate anion , HOBt and the phosphoric acid triamide are split off. The abstraction of the imine hydrogen atom can be done by the OBt anion (shown in the scheme) or directly by the base.
Individual evidence
- ↑ a b c d e J. Coste, D. Le-Nguyen, B. Castro: PyBOP ® : A new peptide coupling reagent devoid of toxic by-product . In: Tetrahedron Lett. . 31, No. 2, 1990, pp. 205-208. doi : 10.1016 / S0040-4039 (00) 94371-5 .
- ↑ a b Data sheet (Benzotriazol-1-yloxy) tripyrrolidinophosphonium hexafluorophosphate, 98% from Sigma-Aldrich , accessed on May 3, 2013 ( PDF ).
- ↑ a b J.-R. Dormoy, B. Castro: The reaction of hexamethyl phosphoric triamide (HMPT) with phosphoryl chloride: A reexamination. Application to a novel preparation of BOP reagent for peptide coupling . In: Tetrahedron Lett. . 20, No. 35, 1979, pp. 3321-3322. doi : 10.1016 / S0040-4039 (01) 95397-3 .
- ^ IA Rivero, R. Somanathan, LH Hellberg: Improved Syntheses of Peptide Coupling Reagents BOP and PyBOP Using Triphosgene . In: Synth. Commun. . 25, No. 14, 1995, pp. 2185-2188. doi : 10.1080 / 00397919508015899 .
- ↑ Sperry, JB; Minteer, CJ; Tao, J .; Johnson, R .; Duzguner, R .; Hawksworth, M .; Oke, S .; Richardson, PF; Barnhart, R .; Bill, DR; Giusto, RA; Weaver, JD: Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing in Org. Process Res. Dev. 22 (2018) 1262-1275, doi : 10.1021 / acs.oprd.8b00193 .
- ↑ E. Frérot, J. Coste, A. Pantaloni, M.-N. Dufour, P. Jouin: PyBOP ® and PyBroP: Two reagents for the difficult coupling of the α, α-dialkyl amino acid, Aib. . In: Tetrahedron . 47, No. 2, 1991, pp. 259-270. doi : 10.1016 / S0040-4020 (01) 80922-4 .
- ↑ T. Høeg-Jensen, CE Olsen, A. Holm: Thioacylation Achieved by Activation of a Monothiocarboxylic Acid with Phosphorus Reagents . In: J. Org. Chem. . 59, No. 6, 1994, pp. 1257-1263. doi : 10.1021 / jo00085a010 .
- ↑ DS Bose, AV Narsaiah: Use of PyBOP as a Convenient Activator for the Synthesis of Nitriles from Primary amide . In: Synthesis . No. 3, 2001, pp. 373-375. doi : 10.1055 / s-2001-11447 .




