Ring narrowing
As a ring contraction , and ring contraction , such chemical reactions are referred to remove in an existing ring, a member or several members. This usually takes place via a cationic intermediate . The ring narrowing is the counterpart to the ring widening .
Reactions
Known ring expansion reactions are, for example
- the Faworski rearrangement of cyclic 2-haloketones,
- the benzilic acid rearrangement of cycloalkane-1,2-dione,
- the Thorpe reaction of dinitriles and
- the rearrangement of cycloheptane to methylcyclohexane by the action of aluminum chloride (AlCl 3 ).
Faworski rearrangement
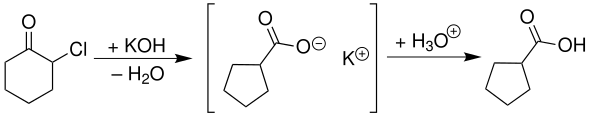
With potassium hydroxide solution, α-chlorocyclohexanone supplies the potassium salt of cyclopentanecarboxylic acid, which can be converted to cyclopentanecarboxylic acid by neutralization :
When an alcoholate is used as the base, α-chlorocyclohexanone is converted into an ester of cyclopentanecarboxylic acid .
Aluminum chloride-induced ring contraction of cycloheptane
When aluminum chloride acts on cycloheptane ( 1 , empirical formula C 7 H 14 ), methylcyclohexane ( 2 , empirical formula C 7 H 14 ) is formed:
Individual evidence
- ↑ Entry on ring reactions. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ^ A b Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 383, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann: Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 376, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann: Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 219, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann: Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 220, ISBN 3-342-00280-8 .
- ↑ A. Faworski, W. Boshowski: Isomeric Transformations of Cyclic α-Monocbloroketones. In: J. Russ. Phys. Chem. 46, 1914, pp. 1097-1102. German version: About isomeric transformations of cyclic α-monochloro ketones. In: Chemisches Zentralblatt . 1915, I, p. 984.
- ^ László Kürti and Barbara Czakó .: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, pp. 164-165, ISBN 978-0-12-429785-2 .