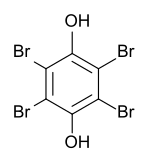
Tetrabromohydroquinone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tetrabromohydroquinone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 2 Br 4 O 2 | ||||||||||||||||||
| Brief description |
gray powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 425.89 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tetrabromohydroquinone is a gray solid that belongs to the group of polyphenols as well as the group of halogen aromatic compounds . The flash point of tetrabromohydroquinone is above 110 ° C.
Occurrence
Tetrabromohydroquinone is found in the gill loach Ptychodera flava and, along with riboflavin, is responsible for its bioluminescence .
presentation
Tetrabromohydroquinone can be obtained by bromination of 1,4-benzoquinone with elemental bromine in glacial acetic acid.
Synthesis is also possible by introducing sulfur dioxide into an ethanolic solution of bromanil :
properties
Tetrabromohydroquinone crystallizes in the monoclinic crystal system in the space group P 2 1 / n (space group no. 14, position 2) with the lattice parameters a = 889.07 pm , b = 473.16 pm, c = 1106.12 pm and β = 92.167 °. In the unit cell are 2 formula units .
Individual evidence
- ↑ a b sheet tetrabromohydroquinone from Acros, accessed on 20 February 2010 .
- ↑ a b c data sheet tetrabromohydroquinone from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ VVRichter: chemistry of carbon compounds .
- ^ A b c Eduard Sarauw: Investigations into benzoquinone and some derivatives of the same , quarterly journal of the Natural Research Society in Zurich, 1881.
- ↑ Tetrabromohydroquinone and riboflavin are possibly responsible for green luminescence in the luminous acorn worm, Ptychodera flava; PMID 15966053 .
- ↑ VR Thalladi, H.-C. Weiss, R. Boese, A. Nangia, G. Desiraju: "A comparative study of the crystal structure of tetrahalogenated hydroquinones and γ-hydroquinone", in: Acta Cryst. , 1999 , B55 , pp. 1005-1013. Full text ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.


