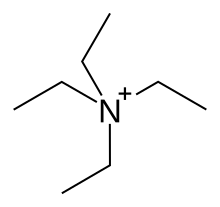
Tetraethylammonium ion
The tetraethylammonium ion is a monovalent cation with the empirical formula C 8 H 20 N + ( semi-structural formula (C 2 H 5 ) 4 N + ). It consists of a nitrogen atom which is tetrahedrally surrounded by four ethyl residues and thus belongs to the group of quaternary ammonium compounds . Formally, it is derived from the ammonium ion by substituting the hydrogen atoms with ethyl radicals. The tetraethylammonium ion is one of the weakly coordinating cations . There are a large number of tetraethylammonium salts of the general formula C 8 H 20 NX, where X is a monovalent anion .
presentation
Tetraethylammonium salts can be prepared by reacting triethylamine with substituted ethyl compounds, the substituent being a leaving group , which then usually functions as a counterion of the salt.
- Reaction of triethylamine with ethyl iodide to form tetraethylammonium iodide .
However, the counterion can also be exchanged afterwards:
- Reaction of tetraethylammonium bromide with silver tetrafluoroborate to give tetraethylammonium tetrafluoroborate with precipitation of silver bromide .
use
It is a neurotoxin that u. a. used in neurophysiology to block the potassium channels in experiments on neurons . TEA blocks the voltage-activated potassium channels that are required for repolarization of the membrane potential after an action potential .
Salts
Tetraethylammonium forms salts with anions . Important ones include:
- Tetraethylammonium bromide
- Tetraethylammonium chloride
- Tetraethylammonium cyanide
- Tetraethylammonium hydroxide
- Tetraethylammonium iodide
- Tetraethylammonium nitrate
- Tetraethylammonium perfluorooctanesulfonate
literature
- Peter Deetjen: Physiology. 4th edition. Urban & Fischer, 2007, ISBN 978-3-437-44440-1 , p. 15. ( limited preview in Google book search)
Individual evidence
- ^ KO Christe, WW Wilson: Reaction of the fluoride anion with acetonitrile. Chloroform and methylene chloride. In: J. Fluorine Chem. 47, 1990, pp. 117-120
- ^ FM Menger, UV Venkataram: Proximity as a component of organic reactivity. In: J. Am. Chem. Soc. 107, 1985, pp. 4706-4709.
- ↑ K. Fukui, K. Ohkubo, T. Yamabe: The Catalytic Activity of Onium Compounds in the Homogeneous Liquid Phase Oxidation of Cumene and α-Pinene. In: Bull. Chem. Soc. Jpn. 42, 1969, pp. 312-318.
- ↑ B. Roux: Extracellular blockade of potassium channels by TEA +: the tip of the iceberg? In: J Gen Physiol . 128 (6), Dec 2006, pp. 649-657. PMID 17130517
- ↑ External identifiers or database links for tetraethylammonium bromide : CAS number: 71-91-0, EC number: 200-769-4, ECHA InfoCard: 100.000.700 , GESTIS substance database : 492379 , PubChem : 6285 , ChemSpider : 6048 , Wikidata : Q5961420 .
- ↑ External identifiers or database links for tetraethylammonium cyanide: CAS number: 13435-20-6, EC number: 236-566-2, ECHA InfoCard: 100.033.228 , Wikidata : Q83026984 .
- ↑ External identifiers or database links for tetraethylammonium hydroxide: CAS number: 77-98-5, EC number: 201-073-3, ECHA InfoCard: 100.000.977 , GESTIS substance database : 492413 , PubChem : 6509 , ChemSpider : 6263 , Wikidata : Q27288030 .
- ↑ External identifiers or database links for tetraethylammonium iodide: CAS number: 68-05-3, EC number: 200-676-9, ECHA InfoCard: 100.000.615 , PubChem : 6225 , ChemSpider : 5990 , Wikidata : Q7706337 .
- ↑ External identifiers or database links for tetraethylammonium nitrate: CAS number: 1941-26-0, EC number: 217-725-5, ECHA InfoCard: 100.016.114 , Wikidata : Q72504985 .
- ↑ External identifiers or database links for tetraethylammonium perfluorooctane sulfonate: CAS number: 56773-42-3, EC number: 260-375-3, ECHA InfoCard: 100.054.869 , GESTIS substance database : 146734 , PubChem : 92531 , ChemSpider : 83538 , Wikidata : Q72481197 .