Tetraethylammonium chloride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
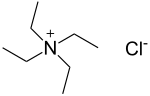
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tetraethylammonium chloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 20 ClN | ||||||||||||||||||
| Brief description |
colorless to light yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 165.71 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.08 g cm −3 (tetrahydrate) |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
soluble in water, ethanol , chloroform or acetone |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tetraethylammonium chloride is a chemical compound from the group of quaternary ammonium compounds .
Extraction and presentation
Tetraethylammonium chloride can be obtained by reacting triethylamine and ethyl chloride or by reacting tetraethylammonium iodide and mercury (I) chloride .
properties
Tetraethylammonium chloride is a colorless to light yellow solid that is soluble in water, ethanol, chloroform or acetone. The compound is amphiphilic . It has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) .
use
Tetraethylammonium chloride is used as a catalyst. It can also be used as a source of tetraethylammonium ions for various pharmaceutical studies and has the ability to block K + channels in various tissues. Tetraethylammonium chloride can also block the transmission of nerve impulses through autonomic ganglia.
Individual evidence
- ↑ a b c Datasheet Tetraethylammonium chloride, for electrochemical analysis, ≥99.0% from Sigma-Aldrich , accessed on November 2, 2018 ( PDF ).
- ↑ a b c d e f g Datasheet Tetraethylammonium chloride from AlfaAesar, accessed on November 2, 2018 ( PDF )(JavaScript required) .
- ↑ a b Z: Römpp Lexikon Chemie, 10th edition, 1996-1999 Volume 6: T - Z . Georg Thieme Verlag, 2014, ISBN 978-3-13-200071-1 ( limited preview in Google book search).
- ↑ Arthur A. Vernon, Gershon M. Goldberg, John H. LaRochelle: The Solubility of Tetraethylammonium Chloride in Benzene-Ethylene Dichloride Mixtures. In: Journal of the American Chemical Society . 73, 1951, p. 2844, doi : 10.1021 / ja01150a122 .
- ↑ Yoshikata Koga, Fumie Sebe, Keiko Nishikawa: Effects of Tetramethyl- and Tetraethylammonium Chloride on H 2 O: Calorimetric and Near-Infrared Spectroscopic Study. In: Journal of Physical Chemistry B . 117, 2013, p. 877, doi : 10.1021 / jp3082744 .
- ↑ RJ Staples: Crystal structure of anhydrous tetraethylammonium chloride, [(CH 3 CH 2 ) 4 N] Cl. In: Journal of Crystallography - New Crystal Structures . 214, 1999, doi : 10.1515 / ncrs-1999-0241 .
