Thianthren
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
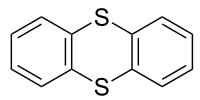
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Thianthren | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 8 S 2 | |||||||||||||||
| Brief description |
beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 216.32 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
151-155 ° C |
|||||||||||||||
| boiling point |
364-366 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Thianthrene is a sulfur containing heterocyclic compound . It is the sulfur analogue of dibenzodioxin . In contrast to this, thianthrene is not planar, but rather has an angle of 128 ° between the planes of the two benzene ring substructures.
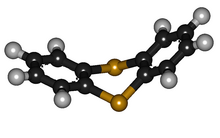 In contrast to the oxygen analogue dibenzodioxin, thianthrene is not planar
In contrast to the oxygen analogue dibenzodioxin, thianthrene is not planar
presentation
Thianthrene can be prepared by reacting disulfur dichloride with benzene in the presence of aluminum chloride .
history
The synthesis of thianthrene was first achieved by John Stenhouse . Thianthrene reacts with sulfuric acid to form a red product. R. Wizinger published the structure of the cation responsible for the color in 1929. Four studies on the structure of the thianthrene radical cation were published independently of one another.
Individual evidence
- ↑ a b c d e f Thianthrene data sheet from Sigma-Aldrich , accessed on January 23, 2011 ( PDF ).
- ^ S. Hosoya: Molecular shapes of thianthrene and related heterocyclic compounds . In: Acta Crystallographica . 16, 1963, pp. 310-312. doi : 10.1107 / S0365110X63000797 .
- ^ KL Gallaher, SH Bauer: Structure and inversion potential of thianthren . In: Journal of the Chemical Society, Faraday Transactions 2 . 71, 1975, pp. 1173-1182. doi : 10.1039 / F29757101173 .
- ↑ MJ Aroney, RJW Le Fèvre, JD Saxby: polaris ability 92. Molecular. The apparent conformations of thianthren and of three of its oxides as solutes in benzene . In: Journal of the Chemical Society (Resumed) . 1965, pp. 571-575. doi : 10.1039 / JR9650000571 .
- ↑ Patent Process for the manufacture of thianthrene , Number 3997560, December 14, 1976.
- ↑ J. Stenhouse: About the products of the dry distillation of the sulfobenzenic acid salts . In: Annals of Chemistry and Pharmacy . 149, 1869, pp. 247-255. doi : 10.1002 / jlac.18691490216 .
- ^ W. Dilthey: Assembly reports Bonner Chemische Gesellschaft , in: Angewandte Chemie , Vol. 42, No. 24, pp. 668–670, June 15, 1929; doi : 10.1002 / anie.19290422405 .
- ^ Gareth R. Eaton: Foundations of Modern EPR. World Scientific, 1998, ISBN 978-9-810-23295-5 , p. 202 ( limited preview in Google book search).