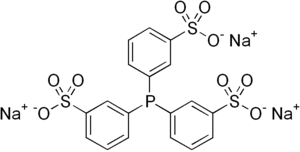
Tri- (sodium-meta-sulfonatophenyl) -phosphine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tri- (sodium-meta-sulfonatophenyl) -phosphine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 12 Na 3 O 9 PS 3 | ||||||||||||||||||
| Brief description |
white, crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 568.42 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tri- (sodium-meta-sulfonatophenyl) -phosphine (abbreviated as TPPTS as an isolated compound or as tppts as a ligand ) is an organic compound with the empirical formula C 18 H 12 Na 3 O 9 PS 3 . It is a white crystalline solid and is an example of a water soluble phosphine. The rhodium hydrido complex of the compound is used as a homogeneous catalyst in the industrial production of butanal by hydroformylation .
synthesis
TPPTS is made by the sulfonation of triphenylphosphine . The sulfonation takes place in the meta position of the phenyl rings. Oleum is used for the sulfonation . The sulfonic acid formed is then mixed with trioctylamine and sodium hydroxide . Immediately after dissolving in the reaction medium, the phosphane is protonated. The resulting phosphonium salt is sulfonated. The gross equation is:
As a Lewis base , TPPTS is stronger than triphenylphosphine .
Use in hydroformylation
Organometallic complexes of the tppts are soluble in water and thus allow heterogenization in homogeneous catalysis. This is the basis for their industrial application. Tppts-modified rhodium catalysts have been used since 1984 in the two-phase hydroformylation of propene by the Ruhrchemie / Rhône-Poulenc process. In hydroformylation, also known as oxo synthesis, an alkene reacts with carbon monoxide and hydrogen to form an aldehyde. Traditionally, hydroformylation was carried out with organometallic rhodium and cobalt complexes in non-aqueous solvents.
Individual evidence
- ↑ a b c d data sheet 39538 at AlfaAesar, accessed on June 17, 2012 ( PDF )(JavaScript required) .
- ↑ Herrmann, WA; Kohlpaintner, CW: Synthesis of Water-Soluble Phosphines and Their Transition Metal Complexes . In: Inorganic Syntheses . 32, 1998, pp. 8-25, doi : 10.1002 / 9780470132630.ch2 .
- ^ Boy Cornils , Richard W. Fischer, Christian Kohlpaintner "Butanals" in Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi : 10.1002 / 14356007.a04_447


