Ubenimex
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
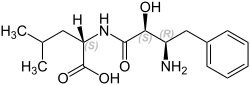
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Ubenimex | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 16 H 24 N 2 O 4 | |||||||||||||||||||||
| Brief description |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 308.378 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
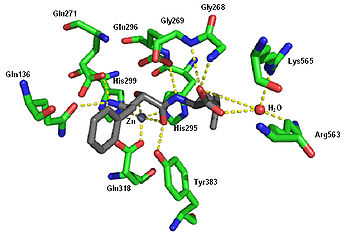
Ubenimex (also Bestatin ) is a competitive , reversible protease inhibitor that inhibits the following enzymes:
- Arginine aminopeptidase ( aminopeptidase B )
- Leukotriene A4 hydrolase (a metalloprotease that functions as both an epoxide hydrolase and an aminopeptidase )
- Alanine aminopeptidase (aminopeptidase M / N)
- Leucine / cystine aminopeptidase (oxytocinase / vasopressinase)
- Membrane dipeptidase (leukotriene D 4 hydrolase)
Ubenimex has long been used in Japan under the trade name Bestatin for the supportive treatment of acute myeloid leukemia . The preparation was launched there in 1987 and can be used orally . Its use in the experimental therapy of lymphedema was new, although this did not meet expectations. For the treatment of pulmonary arterial hypertension (PAH), Ubimex was granted orphan drug status for the EU in 2016 .
Its structure is based on a naturally occurring substance from the bacterium Streptomyces olivoreticuli . Ubenimex inhibits the enzymatic breakdown of oxytocin , vasopressin , enkephalins and various other peptides and substances.
Web links
Individual evidence
- ↑ a b c Ubenimex , Japanese Pharmacopoeia, 17th edition (2016), PMDA , English version, p. 1741.
- ↑ a b The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, ISBN 978-0-911910-00-1 .
- ↑ a b c d data sheet Bestatin hydrochloride, 98% from AlfaAesar, accessed on April 3, 2019 ( PDF )(JavaScript required) .
- ↑ Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T: Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes . In: The Journal of Antibiotics (Tokyo) . tape 29 , no. 1 , 1976, p. 97-99 ( jst.go.jp ).
- ↑ Muskardin DT, Voelkel NF, Fitzpatrick FA: Modulation of pulmonary leukotriene formation and perfusion pressure by Bestatin, an inhibitor of leukotriene A4 hydrolase . In: Biochemical Pharmacology . tape 48 , no. 1 , 1994, p. 131-37 , doi : 10.1016 / 0006-2952 (94) 90232-1 .
- ↑ Sekine K, Fujii H, Abe F: Induction of apoptosis by Bestatin (ubenimex) in human leukemic cell lines . In: Leukemia . tape 13 , no. 5 , 1999, p. 729-34 , doi : 10.1038 / sj.leu.2401388 .
- ↑ Nakanishi Y, Nomura S, Okada M, Ito T, Katsumata Y, Kikkawa F, Hattori A, Tsujimoto M, Mizutani S: Immunoaffinity purification and characterization of native placental leucine aminopeptidase / oxytocinase from human placenta . In: Placenta . tape 21 , no. 7 , 2000, pp. 628-34 , doi : 10.1053 / plac.2000.0564 , PMID 10985965 .
- ↑ Naruki M, Mizutani S, Goto K, Tsujimoto M, Nakazato H, Itakura A, Mizuno K, Kurauchi O, Kikkawa F, Tomoda Y: Oxytocin is hydrolyzed by an enzyme in human placenta that is identical to the oxytocinase of pregnancy serum . In: Peptides . tape 17 , no. 2 , 1996, p. 257-61 , doi : 10.1016 / 0196-9781 (95) 02124-8 , PMID 8801531 .
- ↑ Hirayama Y, Sakamaki S, Takayanagi N, Tsuji Y, Sagawa T, Chiba H, Matsunaga T, Niitsu Y: [Chemotherapy with ubenimex corresponding to patient age and organ disorder for 18 cases of acute myelogeneous leukemia in elderly patients - effects, complications and long-term survival] . In: Gan To Kagaku Ryoho [Cancer & Chemotherapy] . tape 30 , no. 8 , 2003, p. 1113-8 , PMID 12938265 .
- ^ Pharmaceuticals - Introduction of Main Products , Nippon Kayaku; accessed on November 22, 2019.
- ↑ Tian W, Rockson SG, Jiang X, Kim J, Begaye A, Shuffle EM, Tu AB, Cribb M, Nepiyushchikh Z, Feroze AH, Zamanian RT, Dhillon RT, Voelkel NF, Peters-Golden M, Kitajewski J, Dixon JB , Nicolls MR: Leukotriene B4 antagonism ameliorates experimental lymphedema . In: Science Translational Medicine . tape 9 , no. 389 , 2017, p. eaal3920 , doi : 10.1126 / scitranslmed.aal3920 , PMID 28490670 .
- ↑ Eiger Biopharmaceuticals: Eiger BioPharmaceuticals Announces Phase 2 ULTRA Results of Ubenimex in Lower Leg Lymphedema: Study Did Not Meet Primary or Secondary Endpoint. Retrieved April 3, 2019 .
- ↑ Implementation decision of the Commission of March 21, 2016 on the designation of the drug "Ubenimex" as a medicinal product for orphan diseases in accordance with Regulation (EC) No. 141/2000 of the European Parliament and the Council .
- ↑ Bauvois B, Dauzonne D: Aminopeptidase-N / CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects . In: Medicinal Research Reviews . tape 26 , no. 1 , 2006, p. 88-130 , doi : 10.1002 / med.20044 , PMID 16216010 .
- ↑ Protein Data Bank : 1HS6 .