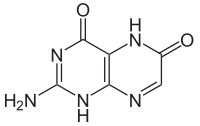
Xanthopterin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Xanthopterin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 5 N 5 O 2 | |||||||||||||||
| Brief description |
orange-yellow crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 179.1362 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
> 360 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Xanthopterin is a heterocyclic compound that is derived from pterin . It occurs as a yellow pterin pigment z. B. in butterfly wings, from which Frederick Gowland Hopkins extracted such dyes for the first time in 1889 . Heinrich Otto Wieland and Clemens Schöpf isolated xanthopterin in its pure form from the wings of the lemon moth in 1924 . The structure of xanthopterin was only clarified in 1940 by Robert Purrmann .
Xanthopterin is also common in other insects and organisms; so it is found in wasps and in human urine. Various microorganisms can produce folic acid from xanthopterin .
See also: Phototrophy # Phototrophic Opisthokonta , paragraph: Phototrophy with Xanthopterin
properties
A crystal structure analysis of xanthopterin is not yet available because of the difficulty in growing suitable crystals. In contrast, xanthopterin hydrochloride could be examined by X-ray: it shows the depicted 4,6-dioxo structure, with protonation taking place at N (3).
Xanthopterin is relatively easily oxidized, with oxygenation at C-7 producing leucopterin .
Syntheses
Purrmann obtained xanthopterin from 2,4,5-triamino-6-hydroxypyrimidine and dichloroacetic acid in two synthesis steps, Koschara in one step with glyoxylic acid .
Individual evidence
- ↑ a b c d e entry on xanthopterin. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Frederick Gowland Hopkins: Note on a yellow pigment in butterflies. In: Proc. Chem. Soc. , Vol. 5 (1889), p. 117, ISSN 0269-3135 .
- ↑ Heinrich Otto Wieland, Clemens Schöpf, in: Ber. German Chem. Ges. , Jg. 58 (1925), p. 2178, ISSN 0365-9488 .
- ↑ Robert Purrmann: About the wing pigments of butterflies. VII. Synthesis of Leucopterin and Nature of Guanopterin . In: Liebig's annals . tape 544 , 1940, ISSN 0075-4617 , pp. 182-190 , doi : 10.1002 / jlac.19405440111 .
- ↑ Jost H. Bieri et al. a .: Helv. Chim. Acta , Vol. 59 (1976), No. 7, pp. 2374-2379, ISSN 0018-019X .
- ↑ Robert Purrmann: The synthesis of Xanthopterin. About the wing pigments of the butterflies. X³ . In: Liebig's annals . tape 546 , no. 1-2 , 1941, ISSN 0075-4617 , pp. 98 , doi : 10.1002 / jlac.19415460107 .
- ^ Walter Koschara: Hoppe-Seylers Zeitschrift für Physiologische Chemie , Vol. 277 (1943), p. 159, ISSN 0018-4888 .