Xylidines
The xylidines (also called aminoxylenes or dimethylanilines ) are aromatic amines with the general formula C 6 H 3 (CH 3 ) 2 NH 2 or as a molecular formula C 8 H 11 N, of which there are six constitutional isomers . They can be as amino - derivatives of xylenes interpret. They arise u. a. as by-products of the fractional distillation of coal tar .
Structure and properties
The xylidines are usually yellowish liquids (with the exception of the solid 3,4-xylidine) and have an amine-like odor. They change color when exposed to light and in contact with air. They are sparingly soluble in water, soluble in ethanol and ether. Xylidines are mostly toxic.
| Xylidines | |||||||||||||||
| Surname | 2,3-xylidine | 2,4-xylidine | 2,5-xylidine | 2,6-xylidine | 3,4-xylidine | 3,5-xylidine | |||||||||
| other names | 2,3-dimethylaniline, 1-amino- 2,3-dimethylbenzene |
2,4-dimethylaniline, 1-amino- 2,4-dimethylbenzene |
2,5-dimethylaniline, 1-amino- 2,5-dimethylbenzene |
2,6-dimethylaniline, 1-amino- 2,6-dimethylbenzene |
3,4-dimethylaniline, 1-amino- 3,4-dimethylbenzene |
3,5-dimethylaniline, 1-amino- 3,5-dimethylbenzene |
|||||||||
| Structural formula |

|

|

|

|

|
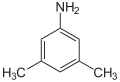
|
|||||||||
| CAS number | 87-59-2 | 95-68-1 | 95-78-3 | 87-62-7 | 95-64-7 | 108-69-0 | |||||||||
| 1300-73-8 (mixture of isomers) | |||||||||||||||
| PubChem | 6893 | 7250 | 7259 | 6896 | 7248 | 7949 | |||||||||
| ECHA InfoCard | 100.001.596 | 100.002.219 |
100.002.229 ( 100.208.101 ) |
100.001.599 | 100.002.217 | 100.003.280 | |||||||||
| 100.013.720 (mixture of isomers) | |||||||||||||||
| Molecular formula | C 8 H 11 N | ||||||||||||||
| Molar mass | 121.18 g mol −1 | ||||||||||||||
| Physical state | liquid | firmly | liquid | ||||||||||||
| Brief description | colorless liquids or solids, yellowish to reddish brown when exposed to light and air, with an amine-like odor | ||||||||||||||
| Melting point | 2 ° C | −16 ° C | 15.5 ° C | 11.2 ° C | 51 ° C | 9.8 ° C | |||||||||
| boiling point | 222 ° C | 214 ° C | 218 ° C | 215 ° C | 228 ° C | 220 ° C | |||||||||
|
pK s value (of the conjugate acid BH + ) |
4.70 | 4.89 | 4.53 | 3.95 | 5.17 | 4.91 | |||||||||
| solubility | sparingly soluble in water, soluble in ethanol and ether | ||||||||||||||
|
GHS labeling |
|
|
|
||||||||||||
| H and P phrases | 331-311-301-373-411 | 302-312-332-315-319-335-351-411 | 331-311-301-373-411 | ||||||||||||
| no EUH phrases | no EUH phrases | no EUH phrases | |||||||||||||
|
261-273-280 301 + 310-311 |
273-280-301 + 310-311 |
273-280-302 + 352 304 + 340-308 + 310 |
273-391-501 |
280-273-304 + 340 302 + 352-309 + 310 |
261-273-280-301 + 310-311 | ||||||||||
presentation
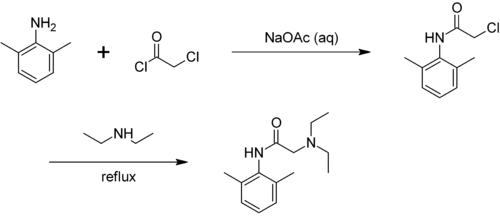
Technically, xylidines are obtained by converting a mixture of isomers of the xylenes . By nitration therefrom forming nitroxylenes from which, by reducing the xylidines are accessible. The product is a mixture of isomers. Hydrochloric acid is added to separate this mixture ; A crystal sludge is deposited, which consists essentially of the hydrochloride of 2,6-xylidine.
use
In general, the xylidines are used in the production of dyes, pesticides and other chemicals. In addition, the 2,3-isomer is used to produce mefenamic acid , the 2,6-isomer is converted into anesthetics (e.g. lidocaine ) and the 3,4-isomer to vitamin B 2 . The technical xylidine is used almost exclusively for the preparation of azo dyes , but it was also used as a component of the rocket fuel Tonka .
Individual evidence
- ↑ Entry for CAS no. 1300-73-8 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c Entry for CAS no. 87-59-2 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c Entry for CAS no. 95-68-1 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c Entry for CAS no. 95-78-3 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c Entry for CAS no. 87-62-7 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c Entry for CAS no. 95-64-7 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c Entry for CAS no. 108-69-0 in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .