Reviparin sodium
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.110.590 |
| | |
Reviparin is an antithrombotic and belongs to the group of low molecular weight heparins (LMWH).
Medical uses
- Prevention of blood clots
- Prophylaxis of perioperative thromboembolism[1][2]
- Treatment of DVT with or without pulmonary embolism (PE)[3]
- Prophylaxis of acute thrombotic events after percutaneous transluminal coronary angioplasty (PTCA)[4][5]
Chemistry
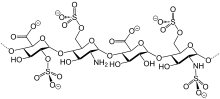
Reviparin is a low molecular weight heparin obtained by nitrous acid depolymerization of heparin extracted from porcine intestinal mucosa. Its structure is characterized, for the most part, by a group of 2-O-sulfo-α-lidopyranosuronic acid. The average molecular weight is about 3900 daltons.
References
- ^ Kakkar VV, Cohen AT, Mohamed MS (1996). "Patients at risk of venous thromboembolism--clinical results with reviparin". Thromb. Res. 81 (2 Suppl): S39–45. doi:10.1016/0049-3848(95)00228-6. PMID 8822126.
- ^ Lassen MR, Backs S, Borris LC, Kaltoft-Sørenson M, Coff-Ganes H, Jeppesen E (1999). "Deep-vein thrombosis prophylaxis in orthopedic surgery: hip surgery". Semin. Thromb. Hemost. 25 Suppl 3: 79–82. PMID 10549720.
- ^ Gore M, Kelkar P, Rege N, Ross C (October 2004). "Reviparin sodium clivarine: a review of its therapeutic use". J Indian Med Assoc. 102 (10): 589–90, 592. PMID 15887830.
- ^ Preisack MB, Karsch KR (December 1993). "Experimental and early clinical experience with reviparin-sodium for prevention of restenosis after percutaneous transluminal coronary angioplasty". Blood Coagul. Fibrinolysis. 4 Suppl 1: S55–8, discussion S59–60. PMID 8180331.
- ^ Karsch KR, Preisack MB, Baildon R, Eschenfelder V, Foley D, Garcia EJ, Kaltenbach M, Meisner C, Selbmann HK, Serruys PW, Shiu MF, Sujatta M, Bonan R (November 1996). "Low molecular weight heparin (reviparin) in percutaneous transluminal coronary angioplasty. Results of a randomized, double-blind, unfractionated heparin and placebo-controlled, multicenter trial (REDUCE trial). Reduction of Restenosis After PTCA, Early Administration of Reviparin in a Double-Blind Unfractionated Heparin and Placebo-Controlled Evaluation". J. Am. Coll. Cardiol. 28 (6): 1437–43. doi:10.1016/s0735-1097(96)00343-9. hdl:1765/55740. PMID 8917255.
External links
- reviparin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Yusuf S, Mehta SR, Xie C, et al. (2005). "Effects of reviparin, a low-molecular-weight heparin, on mortality, reinfarction, and strokes in patients with acute myocardial infarction presenting with ST-segment elevation". JAMA. 293 (4): 427–35. doi:10.1001/jama.293.4.427. PMID 15671427.
