1,1,2,2-tetrabromoethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
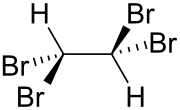
|
||||||||||||||||
| Wedges to clarify the geometry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,1,2,2-tetrabromoethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 2 Br 4 | |||||||||||||||
| Brief description |
red liquid with a sweet, pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 345.67 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
2.97 g cm −3 |
|||||||||||||||
| Melting point |
−1 ° C |
|||||||||||||||
| boiling point |
151 ° C at 72 hPa |
|||||||||||||||
| Vapor pressure |
0.027 hPa (20 ° C) |
|||||||||||||||
| solubility |
heavy in water (0.68 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.6353 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 1 ml m −3 or 14 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,1,2,2-Tetrabromoethane , often the abbreviation TBE , is a brominated hydrocarbon . It arises from the bromination of ethyne with bromine .
properties
Freshly distilled, it is a colorless liquid that, over time, takes on a reddish-brown color due to decomposition reactions in daylight. 1,1,2,2-Tetrabromoethane has an unpleasant, sweet, pungent and very intense odor.
1,1,2,2-Tetrabromoethane is a heavy liquid that is used in mineralogy to separate and determine the density of heavy minerals. There this liquid is also known under the name Muthmann's liquid (after Wilhelm Muthmann , 1899). Due to its high toxicity , 1,1,2,2-tetrabromoethane should no longer be used, especially since there are now considerably less dangerous heavy liquids. TBE is a strong kidney and liver poison.
Individual evidence
- ↑ a b c d e f g Entry on 1,1,2,2-tetrabromoethane in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on 1,1,2,2-tetrabromoethane. In: Römpp Online . Georg Thieme Verlag, accessed on March 2, 2017.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-468.
- ↑ Entry on 1,1,2,2-tetrabromoethane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 79-27-6 or 1,1,2,2-tetrabromoethane ), accessed on November 2, 2015.
- ^ Andreas von Usedom: Organic chemistry, biochemistry, chemical industry . Mentor, 2003, ISBN 978-3-580-64134-4 ( limited preview in Google Book Search).
- ^ S. Gangolli: The Dictionary of Substances and Their Effects: TZ and index . Royal Society of Chemistry, 1999, ISBN 978-0-85404-838-0 , pp. 65 ( limited preview in Google Book search).
