1,3-bis (aminomethyl) cyclohexane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
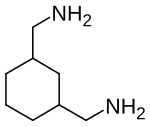
|
||||||||||||||||
| Structural formula without representation of the stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,3-bis (aminomethyl) cyclohexane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 18 N 2 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 142.24 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.945 g cm −3 |
|||||||||||||||
| Melting point |
−25 ° C |
|||||||||||||||
| boiling point |
240 ° C |
|||||||||||||||
| Vapor pressure |
34 Pa (25 ° C) |
|||||||||||||||
| solubility |
very light in water (> 1000 g / l) |
|||||||||||||||
| Refractive index |
1.493 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,3-bis (aminomethyl) cyclohexane (1,3-BAC) is a mixture of three stereoisomeric chemical compounds from the group of aliphatic amines .
When "1,3-bis (aminomethyl) cyclohexane" without in this article or in the scientific literature the name prefix is needed is always a mixture of equal parts of ( S , S ) form and ( R , R ) form and changing proportions of the meso form meant (see section stereochemistry).
presentation
1,3-bis (aminomethyl) cyclohexane can be prepared by hydrogenating various compounds, for example from m- xylylenediamine , from 1,3- benzenedicarbonitrile or from dicyanocyclohexane .
use
1,3-Bis (aminomethyl) cyclohexane is used industrially as a hardener for epoxy resins , as a raw material for the production of polyamides and isocyanates , as a rubber chemical, for paper processing agents , in fiber treatment agents and in cleaning agents.
Stereoisomers
1,3-bis (aminomethyl) cyclohexane contains two equally substituted stereocenters, consequently there are three stereoisomers:
- ( S , S ) -1,3-bis (aminomethyl) cyclohexane
- ( R , R ) -1,3-bis (aminomethyl) cyclohexane
- meso -1,3-bis (aminomethyl) cyclohexane
The technical product is a mixture of these three isomers.
Individual evidence
- ↑ a b c d e f g h i Entry on 1,3-bis (aminomethyl) cyclohexane in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Data sheet 1,3-Cyclohexanebis (methylamine), mixture of isomers, 99% from Sigma-Aldrich , accessed on July 2, 2015 ( PDF ).
- ^ Leslie G. Humber: Agents Affecting Lipid Metabolism. XVI. The Synthesis of Analogs of trans-1,4-Bis (2-chlorobenzylaminomethyl) cyclohexane . In: Journal of Medicinal Chemistry . 8, No. 3, 1965, pp. 401-404. doi : 10.1021 / jm00327a033 .
- ↑ a b Patent EP1586554 : Purification of 1,3-bis (aminomethyl) cyclohexane by distillation. Published on October 19, 2005 , Inventors: Kazuhiko Amakawa, Kuniaki Muneyasu, Hiroshi Watanabe.
- ^ Ha Q. Pham, Maurice J. Marks: Epoxy Resins . In: Ullmann's Encyclopedia of Industrial Chemistry . September, p. 192. doi : 10.1002 / 14356007.a09_547.pub2 .

