1,1-difluoroethene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
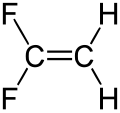
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,1-difluoroethene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 2 F 2 | |||||||||||||||
| Brief description |
extremely flammable, colorless gas with a slightly ethereal odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 64.03 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
−144 ° C |
|||||||||||||||
| boiling point |
−84 ° C |
|||||||||||||||
| Vapor pressure |
3.612 M Pa (20 ° C) |
|||||||||||||||
| solubility |
moderate in water (254 mg l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−335.0 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,1-Difluoroethene is a chemical compound from the group of aliphatic unsaturated fluorocarbons .
Extraction and presentation
There are various manufacturing processes for 1,1-difluoroethene, for example dechlorination of 1,2-dichloro-1,1-difluoroethane or dehydrofluorination of 1,1,1-trifluoroethane . The global production volume in 1999 was around 33,000 tons.
properties
1,1-Difluoroethene is an extremely flammable, colorless gas with a slightly ethereal odor. It is easy to polymerize and is sold in compressed gas cylinders in liquefied form. At higher temperatures it is chemically unstable. The critical temperature is 30.1 ° C, the critical pressure is 44.33 bar, the critical density is 6.51 mol·l −1 and the triple point temperature is −144 ° C (melting temperature).
use
1,1-Difluoroethene is used to make polyvinylidene fluoride and copolymers with chlorotrifluoroethylene and hexafluoropropylene .
safety instructions
1,1-Difluoroethene forms an explosive mixture with air. The explosion range is between 4.7% by volume (125 g / m 3 ) as the lower explosion limit (LEL) and 25.1% by volume (664 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 390 ° C. The substance therefore falls into temperature class T2. During thermal decomposition, hydrogen fluoride is formed.
See also
- 1,2-difluoroethene (in cis and trans form)
- 1-chloro-1,1-difluoroethane
Individual evidence
- ↑ a b c d e f g h Entry on 1,1-difluoroethene in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Ethene, 1,1-difluoro- , accessed on November 4, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-21.
- ↑ a b c Mears, WH; Steel, RF; Orfeo, SR; Shair, RC; Kells, LF; Thompson, W .; McCann, H .: Thermodynamic Properties of Halogenated Ethanes and Ethylenes in Ind. Eng. Chem. 47 (1955) 1449-1954, doi : 10.1021 / ie50547a052 .
- ^ A b E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.

