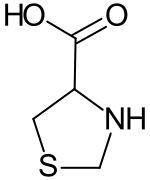
1,3-thiazolidine-4-carboxylic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| Structural formula without information on stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 1,3-thiazolidine-4-carboxylic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 7 NO 2 S | |||||||||||||||||||||
| Brief description |
colorless needles |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 133.17 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
196–197 ° C (decomposition) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
1,3-thiazolidine-4-carboxylic acid is a thiazolidine - derivative having a carboxy substituent in α-position to the amino group . The compound thus belongs to the heterocyclic secondary α- amino acids . The structural element of 1,3-thiazolidine-4-carboxylic acid can be found in a number of active pharmaceutical ingredients , especially penicillins
synthesis
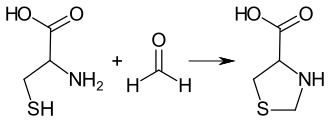
The synthesis of 1,3-thiazolidine-4-carboxylic acid was first described in 1936 by Maxwell P. Schubert and independently thereof in 1937 by Hans Thacher Clarke . The compound is formed when formaldehyde is reacted with an equimolar amount of cysteine in hydrochloric acid. More detailed studies show that formaldehyde is initially added to the thiol group , followed by cyclization with the amino group with elimination of water.
Stereochemistry
1,3-Thiazolidine-4-carboxylic acid is a chiral compound with two enantiomers .
Isomers of 1,3-thiazolidine-4-carboxylic acid Surname (4 S ) -1,3-thiazolidine-4-carboxylic acid (4 R ) -1,3-thiazolidine-4-carboxylic acid other names D- thiazolidine-4-carboxylic acid L- thiazolidine-4-carboxylic acid Structural formula 
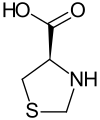
CAS number 45521-09-3 34592-47-7 444-27-9 ( DL ) EC number 256-240-3 252-106-3 207-146-6 ( DL ) ECHA info card 100.051.111 100.047.355 100.006.498 ( DL ) PubChem 198253 93176 9934 ( DL ) DrugBank - DB02846 DB12856 ( DL ) Wikidata Q27133347 Q27093808 Q23637400 ( DL )
Properties and use
1,3-Thiazolidine-4-carboxylic acid has hepatoprotective (liver-protecting), anticancer and anti-aging properties. The compound is used under the name TIMONACIC as an ingredient in cosmetic products for skin care .
Individual evidence
- ↑ Entry on TIMONACIC in the CosIng database of the EU Commission, accessed on August 11, 2020.
- ^ A b c d Sarah Ratner, HT Clarke: The Action of Formaldehyde upon Cysteine . In: Journal of the American Chemical Society . tape 59 , no. 1 , January 1937, p. 200 , doi : 10.1021 / ja01280a050 .
- ↑ a b Safety data sheet: Timonacic. (PDF) July 24, 2017, accessed on August 11, 2020 .
- ^ Maxwell P. Schubert: Compounds of Thiol Acids with Aldehydes . In: Journal of Biological Chemistry . tape 114 , 1936, pp. 341 ( PDF ).
- ↑ Th. Wieland ao: Methods for the production and conversion of amino acids and derivatives . In: Eugen Müller (Ed.): Houben-Weyl - Methods of Organic Chemistry . 4th edition. tape XI / 2 . Thieme, Stuttgart 1958, p. 439 f . ( limited preview in Google Book search).
- ↑ Yat-Hing Ham, KK Jason Chan, Wan Chan: Thioproline Serves as an Efficient Antioxidant Protecting Human Cells from Oxidative Stress and Improves Cell Viability . In: Chemical Research in Toxicology . tape 33 , no. 7 , July 20, 2020, p. 1815 , doi : 10.1021 / acs.chemrestox.0c00055 .