1-naphthoic acid chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
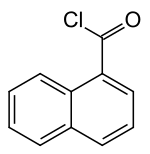
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-naphthoic acid chloride | |||||||||||||||
| other names |
1-naphthoyl chloride |
|||||||||||||||
| Molecular formula | C 11 H 7 ClO | |||||||||||||||
| Brief description |
brown liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 190.63 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.265 g cm −3 |
|||||||||||||||
| Melting point |
16-19 ° C |
|||||||||||||||
| boiling point |
190 ° C (47 hPa) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1-naphthoic acid chloride is a naphthalene derivative . It belongs to the polycyclic aromatic hydrocarbons (PAH) class of substances .
synthesis
1-naphthoic acid chloride can be prepared from 1-naphthoic acid with phosphorus pentachloride .
properties
It is a corrosive liquid. As a condensed aromatic, 1-napthoic acid chloride shows fluorescence .
use
- 1-Naphthoic acid chloride is used as a derivatizing reagent for the analysis of dodecyl alcohols in water samples.
- The compound is important as a fluorescence marker for HPLC analyzes.
- Starting from 1-naphthoic acid chloride, 1-naphthylacetic acid can be prepared in the sense of an Arndt-Eistert synthesis .
- The acid chloride of naphthoic acid is used to prepare 2-ethyl-1-pentyl-3- (1-naphthoyl) indole, an indole derivative with a cannabinoid mimetic effect.
Individual evidence
- ↑ a b c d e f data sheet 1-naphthoic acid chloride, 97% from Sigma-Aldrich , accessed on November 10, 2018 ( PDF ).
- ↑ A. Zgoła-Grześkowiak, T. Grześkowiak: Solid-phase extraction combined with dispersive liquid-liquid microextraction, fast derivatisation and high performance liquid chromatography-tandem mass spectrometry analysis for trace determination of short-chained dodecyl alcohol ethoxylates and dodecyl alcohol in environmental water samples. J. Chromatogr. A , 2012 , 1251. DOI: 10.1016 / j.chroma.2012.06.056
- ↑ Vincenzo Lippolis, Michelangelo Pascale a. a .: Improvement of detection sensitivity of T-2 and HT-2 toxins using different fluorescent labeling reagents by high-performance liquid chromatography ☆. In: Talanta . 74, 2008, p. 1476, doi : 10.1016 / j.talanta.2007.09.024 .
- ↑ Toyohiko Aoyama, Takayuki Shioiri: New methods and reagents in organic synthesis. 8. Trimethylsilyldiazomethanes. A new, stable, and safe reagent for the classical arndt-eistert synthesis. In: Tetrahedron Lett. 21, 1980, p. 4461, doi : 10.1016 / S0040-4039 (00) 92200-7 .
- ↑ JW Huffman, R. Mabon, MJ Wu, J. Lu, R. Hart, DP Hurst, PH Reggio, JL Wiley, BR Martin: 3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence aromatic for stacking interactions with the CB (1) cannabinoid receptor. In: Bioorg. Med. Chem. 11, 2003, pp. 539-549, PMID 12538019 .
