1-vinylhexahydro-2 H -azepin-2-one
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
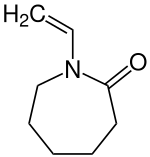
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-vinylhexahydro-2 H -azepin-2-one | |||||||||||||||
| other names |
N- vinyl caprolactam |
|||||||||||||||
| Molecular formula | C 8 H 13 NO | |||||||||||||||
| Brief description |
yellowish solid with a faint odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 139.20 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.01 g cm −3 (40 ° C) |
|||||||||||||||
| Melting point |
34 ° C |
|||||||||||||||
| boiling point |
113–116 ° C (13 hPa) |
|||||||||||||||
| Vapor pressure |
<0.1 hPa (20 ° C) |
|||||||||||||||
| solubility |
soluble in water (approx. 41 g l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1-Vinylhexahydro-2 H -azepin-2-one is a chemical compound from the group of nitrogen heterocycles with a keto group .
Extraction and presentation
1-Vinylhexahydro-2 H -azepin-2-one can be prepared by reaction of acetylene with caprolactam with potassium hydroxide as catalyst and 18-crown-6-ether are obtained as a cocatalyst.
properties
1-Vinylhexahydro-2 H -azepin-2-one is a flammable, hardly inflammable, yellowish solid with a faint odor, which is soluble in water. Its aqueous solution has an alkaline reaction.
use
1-vinylhexahydro-2 H -azepin-2-one is used as a reactive diluent and is used in a wide range of UV-curing screen printing inks , for fiberglass layers and rapid prototyping, and in UV varnishes and adhesives . It also serves as a building block for the synthesis of paper coatings.
Individual evidence
- ↑ a b c d e f g h i j Entry on 1-vinylhexahydro-2H-azepin-2-one in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Shubo, Feng & Shuyuan, Li & Cunfeng, He & Erli, Zheng & Xinliang, Tang (2009): Synthesis of N-vinyl caprolactam. Catalysis Today 140.169-173, doi: 10.1016 / j.cattod.2008.10.014 .
- ↑ Data sheet N-Vinyl-epsilon-caprolactam, 99% from AlfaAesar, accessed on January 28, 2019 ( PDF )(JavaScript required) .

