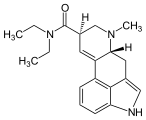
1CP-LSD
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | 1CP-LSD | ||||||
| other names |
|
||||||
| Molecular formula | C 24 H 29 N 3 O 2 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| Drug information | |||||||
| Mechanism of action | |||||||
| properties | |||||||
| Molar mass | 391.5 g mol −1 | ||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
1CP-LSD (1-Cyclopropionyl- D -lysergsäurediethylamid) is a psychedelic acting psychotropic substance and research chemical . 1CP-LSD is a derivative of lysergic acid , which occurs naturally in ergot alkaloids , as well as an analogue of LSD and a homologue of 1P-LSD and ALD-52 .
chemistry
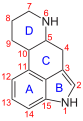
Chemically, 1CP-LSD belongs to the Ergoline structural class . The tetracyclic ergoline is characteristic of the chemical structure of ergot alkaloids . In contrast to LSD, 1CP-LSD has an additional N 1 -cyclopropionyl group. Chemical modifications in the N 1 position are among the most frequently carried out changes to the ergoline system, since the indole nitrogen is easily accessible for alkylations , acylations , Mannich reactions and Michael additions .
Structural formula of ALD-52 (1-acetyl- D- LSD)
Structural formula of 1P-LSD (1-Propionyl- D -LSD)
Structural formula of 1B-LSD (1-butyryl- D -LSD)
pharmacology
It is assumed that 1CP-LSD (like the acyl homologues 1P-LSD and ALD-52 ) is deacylated to LSD in the body by carboxylesterase , which studies of human blood serum have shown.
See also
Web links
- isomerdesign.com: 1CP-LSD (English)
Individual evidence
- ↑ a b c Simon D. Brandt, Pierce V. Kavanagh et al. a .: Return of the lysergamides. Part VI: Analytical and behavioral characterization of 1 ‐ cyclopropanoyl ‐ d ‐ lysergic acid diethylamide (1CP ‐ LSD). In: Drug Testing and Analysis. 2020, doi : 10.1002 / dta.2789 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Petr Bulej, Ladislav Cvak: Chemical modifications of ergot alkaloids . In: Vladimir Kren, Ladislav Cvak (eds.): Ergot: The Genus Claviceps. Medicinal and Aromatic Plants - Industrial Profiles . CRC Press, 2004, ISBN 0203304195 , pp. 202-230.
- ↑ AL Halberstadt, M. Chatha u. a .: Pharmacological and biotransformation studies of 1-acyl-substituted derivatives of d-lysergic acid diethylamide (LSD). In: Neuropharmacology. November 2019, doi : 10.1016 / j.neuropharm.2019.107856 , PMID 31756337 .
- ↑ Lea Wagmann, Lilian HJ Richter a. a .: In vitro metabolic fate of nine LSD-based new psychoactive substances and their analytical detectability in different urinary screening procedures. In: Analytical and Bioanalytical Chemistry . 2019, doi : 10.1007 / s00216-018-1558-9 .