2,2-bis (hydroxymethyl) propionic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,2-bis (hydroxymethyl) propionic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 10 O 4 | ||||||||||||||||||
| Brief description |
white crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 134.13 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.37 g cm −3 |
||||||||||||||||||
| Melting point |
188-191 ° C |
||||||||||||||||||
| solubility |
101 g l −1 (in water) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
When 2,2-bis (hydroxymethyl) propionic acid is a chemical compound having two hydroxy groups and a carboxy group contains. Colloquially, 2,2-bis (hydroxymethyl) propionic acid is better known as DMPA or also dimethylolpropionic acid.
properties
Since 2,2-bis (hydroxymethyl) propionic acid has two different functional groups , it can be used for a wide variety of syntheses. In addition to reaction with other substances, 2,2-bis (hydroxymethyl) propionic acid can also react with itself via esterification .
use
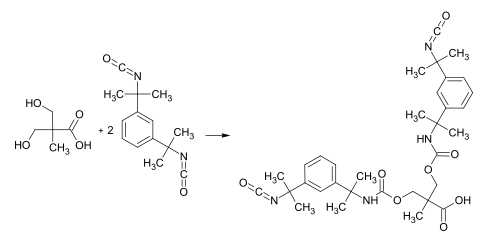
In the paint sector, 2,2-bis (hydroxymethyl) propionic acid is often used as a component to convert actually organically soluble binders into an aqueous dispersion. This is achieved by incorporating 2,2-bis (hydroxymethyl) propionic acid via the hydroxyl groups and then neutralizing the carboxy group. The ionic character of the neutralized carboxy group is sufficient for the binder molecules to arrange themselves into small spheres, similar to micelles . The neutralized carboxy group is now located in the boundary layer between the organic and the aqueous phase. This procedure is used for aqueous UV binders as well as for alkyd and polyester- based binders which can cure via autoxidation or co-crosslinking. The products are generally referred to as aqueous polyurethane dispersions . In the above case i. d. Usually an adduct is produced from a suitable diisocyanate (e.g. IPDI or TMXDI) and 2,2-bis (hydroxymethyl) propionic acid, which can be incorporated into hydroxyl-containing resins via an excess of isocyanate:
In addition to incorporation into a hydroxyl-containing resin, 2,2-bis (hydroxymethyl) propionic acid can also be incorporated into oligomeric isocyanates in order to give them a hydrophilic character. For this purpose, 2,2-bis (hydroxymethyl) propionic acid is first reacted with an isocyanate in excess. The excess isocyanate groups are then blocked with a so-called "blocking agent". Only at higher temperatures, such as a baking process, does the “blocking agent” split off and the isocyanate group is released and can react. This also ensures that the isocyanate group, which is very reactive towards water, can be converted into an aqueous phase without reacting off.
There is also the possibility of using 2,2-bis (hydroxymethyl) propionic acid for the synthesis of dendrimeric molecules, also known as hyperbranched molecules. If one assumes a building block carrying higher functional hydroxyl groups as the core of this molecule and reacts each hydroxyl group with 2,2-bis (hydroxymethyl) propionic acid, the number of hydroxyl groups present in the molecule doubles. If this reaction step is repeated, one more so-called shell is obtained each time; the molecule enlarges. If at the end the hydroxyl groups are reacted with a bifunctional component, dendrimeric UV binders can be produced, for example. Above all, dendrimer molecules bring low solution viscosities and improved technological properties with them. A prerequisite for a successful synthesis is always a selective reaction with as few undesirable side reactions as possible.
Individual evidence
- ↑ a b c d e f Entry on 2,2-bis (hydroxymethyl) propionic acid in the GESTIS substance database of the IFA , accessed on June 30, 2018(JavaScript required) .
- ↑ Ulrich Poth: Synthetic binders for coating systems . Vincentz Network, Hannover 2016, ISBN 978-3-86630-611-0 , p. 80, 222, 361 .
- ↑ Ulrich Poth: Polyester and alkyd resins: Basics and applications . Vincentz Network, Hannover 2014, ISBN 978-3-86630-663-9 , p. 113, 143, 206 .
- ↑ Walter Krauß: Binder for solvent-based and solvent-free systems . 2., ext. and rework. Ed. Hirzel, Stuttgart a. a. 1998, ISBN 3-7776-0886-6 , pp. 254 .
- ↑ Ulrich Poth, Bodo Müller: Paint formulation and paint recipe: the textbook for training and practice . Vincentz, Hannover 2003, ISBN 3-87870-746-0 , p. 168 .
- ↑ Bernd Strehmel, Peter Mischke, Michael Groteklaes, Thomas Brock: Textbook of lacquer technology . 4th revised edition. Vincentz Network, [Place of publication not identified], ISBN 3-86630-815-9 , pp. 93 .
- ↑ Ulrich Poth: Synthetic binders for coating systems . Vincentz Network, Hannover 2016, ISBN 978-3-86630-611-0 , p. 247 .
- ↑ Roland Baumstark, Reinhold Schwalm, Manfred Schwartz, Ulrich Poth: Acrylate resins . Vincentz Network, Hannover 2014, ISBN 978-3-86630-820-6 , p. 118 .
- ↑ Roland Baumstark, Reinhold Schwalm, Manfred Schwartz, Ulrich Poth: Acrylate resins . Vincentz Network, Hannover 2014, ISBN 978-3-86630-820-6 , p. 300 .
- ↑ Ulrich Poth: Synthetic binders for coating systems . Vincentz Network, Hannover 2016, ISBN 978-3-86630-611-0 , p. 83-85 .