2,3,5-trimethyl- p -benzoquinone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
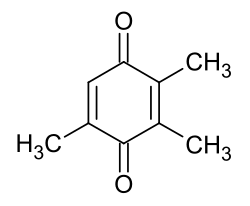
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,3,5-trimethyl- p -benzoquinone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 10 O 2 | ||||||||||||||||||
| Brief description |
yellowish to reddish solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 150.18 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.08 g cm −3 |
||||||||||||||||||
| Melting point |
32 ° C |
||||||||||||||||||
| boiling point |
215.3 ° C |
||||||||||||||||||
| Vapor pressure |
2.5 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,3,5-Trimethyl- p -benzoquinone is a chemical compound from the group of ketones . It is an alkyl-substituted 1,4-benzoquinone .
Extraction and presentation
2,3,5-Trimethyl- p -benzoquinone can be obtained by oxidizing 2,3,5-trimethylphenol with Fremy's salt .
properties
2,3,5-Trimethyl- p -benzoquinone is a yellowish to reddish solid that is sparingly soluble in water.
use
2,3,5-Trimethyl- p -benzoquinone is an important intermediate for the production of trimethylhydroquinone , an essential intermediate for the synthesis of α-tocopherol (vitamin E).
Individual evidence
- ↑ a b c d Entry on 2,3,5-Trimethylcyclohexa-2,5-diene-1,4-dione> 98.0% at TCI Europe, accessed on January 19, 2020.
- ↑ a b c d e f g h i j k Entry on 2,3,5-trimethyl-p-benzoquinone in the GESTIS substance database of the IFA , accessed on January 19, 2020(JavaScript required) .
- ^ Emil Fürer: About the conversion of vitamin E in the animal body . Juris-Verlag, 1963, OCLC 1070921882 , p. 45 ( limited preview in Google Book search).
- ↑ a b Google Patents: EP0387820A2 - Process for the preparation of 2,3,5-trimethyl-p-benzoquinone - Google Patents , accessed on January 19, 2020


