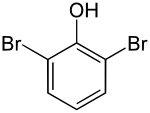
2,6-dibromophenol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,6-dibromophenol | |||||||||||||||
| Molecular formula | C 6 H 4 Br 2 O | |||||||||||||||
| Brief description |
whitish crystalline powder with a phenolic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 251.9 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
56-57 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| pK s value |
6.67 (25 ° C) |
|||||||||||||||
| solubility |
slightly soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,6-Dibromophenol is a chemical compound that belongs to both the phenols and the halogen aromatic compounds .
Occurrence in nature
2,6-Dibromophenol was found in the sea worms Balanoglossus biminiensis .
Derivatives
The methyl ether can be prepared by methylation with dimethyl sulfate and is also known under the common name 2,6-dibromanisole . Its melting point is 13 ° C, its boiling point at 34 mmHg at 143-145 ° C.
The ethyl ether ( 2,6-dibromophenetol ) melts at 40.5 ° C.
Esterification with acetic anhydride yields the acetate, which melts at 46 ° C (CAS number: 28165-72-2).
Further bromination of 2,6-dibromophenol with bromine in potassium bromide solution gives 2,4,6-tribromophenol , which in turn reacts further with bromine to form 2,4,4,6-tetrabromo-2,5-cyclohexadienone. This reaction can be reversed by hydrogen iodide .
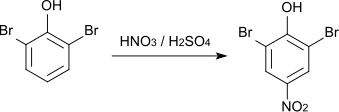
The nitration of 2,6-dibromophenol with nitric acid yields 2,6-dibromo-4-nitrophenol (CAS number: 99-28-5, melting point 144–145 ° C).
Individual evidence
- ↑ a b c data sheet 2,6-dibromophenol from AlfaAesar, accessed on August 2, 2010 ( PDF )(JavaScript required) .
- ↑ a b c d e f g Dictionary of organic compounds, p. 1971 ( limited preview in the Google book search).
- ↑ a b data sheet 2,6-dibromophenol from Sigma-Aldrich , accessed on March 19, 2011 ( PDF ).
- ↑ John A. Price: "The Structure of Tribromophenol bromide", in: J. Am. Chem. Soc. , 1955 , 77 (20), pp. 5436-5437; doi : 10.1021 / ja01625a081 .
- ↑ Hans P. Latscha, Helmut A. Klein, Gerald W. Linti: "Analytical Chemistry: Chemistry-Basiswissen III", p. 287 ( limited preview in the Google book search).
- ^ FG Pope, AS Wood: "CXCIII.-The bromination of phenol. 2: 4- and 2: 6-dibromophenol" in J. Chem. Soc., Trans. 1912 , 101 , pp. 1823-1829. doi : 10.1039 / CT9120101823
- ^ WW Hartman, JB Dickey: 2,6-Dibromo-4-nitrophenol In: Organic Syntheses . 15, 1935, p. 6, doi : 10.15227 / orgsyn.015.0006 ; Coll. Vol. 2, 1943, p. 173 ( PDF ).