2-bromobutane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
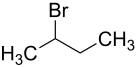
|
||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-bromobutane | |||||||||||||||
| other names |
sec-butyl bromide |
|||||||||||||||
| Molecular formula | C 4 H 9 Br | |||||||||||||||
| Brief description |
Flammable, volatile, colorless liquid with an aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 137.02 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.26 g cm −3 |
|||||||||||||||
| Melting point |
−112 ° C |
|||||||||||||||
| boiling point |
91 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.437 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Bromobutane is a chemical compound from the group of aliphatic halogenated hydrocarbons and organic bromine compounds . It is isomeric to 1-bromobutane , isobutyl bromide and tert-butyl bromide and belongs to the group of butyl bromides .
Isomers
It occurs in two enantiomeric forms, ( R ) -2-bromobutane and ( S ) -2-bromobutane.
| Isomers of 2-bromobutane | ||
| Surname | ( S ) -2-bromobutane | ( R ) -2-bromobutane |
| other names | (+) - 2-bromobutane | (-) - 2-bromobutane |
| Structural formula |  |

|
| CAS number | 5787-32-6 | 5787-33-7 |
| 78-76-2 (unspec.) | ||
| EC number | - | - |
| 201-140-7 (unspec.) | ||
| ECHA info card | - | - |
| 100.001.037 (unspec.) | ||
| PubChem | 12236140 | 637147 |
| 6554 (unspec.) | ||
| Wikidata | Q27254482 | Q27252707 |
| Q209314 (unspec.) | ||
Extraction and presentation
2-Bromobutane can be obtained by reacting 1-butene , 2-butene or 2-butanol with hydrogen bromide .
properties
2-bromobutane forms highly flammable vapor-air mixtures. The compound has a flash point of 21 ° C. The explosion range is between 2.6 vol.% As the lower explosion limit (LEL) and 6.6 vol.% As the upper explosion limit (UEL). The ignition temperature is 265 ° C. The substance therefore falls into temperature class T3.
use
2-bromobutane is used in the manufacture of medicines and fragrances (to introduce the butyl group ).
Web links
- uni-regensburg.de: Reaction of the isomeric butyl bromides with AgNO 3 (learning objective: nucleophilic substitution - SN 1 and SN 2 ) by Peter Keusch.
Individual evidence
- ↑ a b c d e f g h i j k l m n o Entry on 2-bromobutane in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Data sheet 2-bromobutane (PDF) from Merck , accessed on July 9, 2010.
- ↑ 2-Bromobutane data sheet from Sigma-Aldrich , accessed on July 22, 2013 ( PDF ).
- ^ Francis A. Carey; Organic Chemistry , ISBN 0-07-290501-8 , pp. 215, p. 275.
- ↑ a b c E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.


