3,3'-dichlorobenzidine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
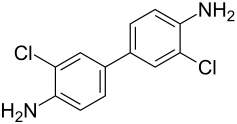
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3,3'-dichlorobenzidine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 10 Cl 2 N 2 | ||||||||||||||||||
| Brief description |
colorless or light brown to purple, flammable crystal needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 253.13 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
132-133 ° C |
||||||||||||||||||
| boiling point |
368 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 0.003 ml m −3 or 0.03 mg m −3 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
3,3'-dichlorobenzidine is a chemical compound from the group of chlorinated benzidine derivatives .
Extraction and presentation
3,3'-dichlorobenzidine is by reduction of o -Nitrochlorbenzol obtained to the corresponding Hydrazobenzolderivat and subsequent rearrangement.
properties
In pure form it is available as a colorless solid, and as a technical product as light brown to purple-tinged needles. It is also often sold as a dihydrochloride . Thermal decomposition produces nitrous gases and hydrogen chloride.
use
3,3'-dichlorobenzidine is used on a large scale as a diazotization component for the production of azo pigments (e.g. diarylide pigments , Pigment Yellow 12 , Pigment Yellow 13 ). Today, 3,3′-dichlorobenzidine is no longer produced in Germany. After reductive cleavage of azo groups, the substance must not be released from textiles or leather products that come into direct contact with human skin for a longer period of time (Appendix 1 of the Consumer Goods Ordinance ).
safety instructions
3,3′-dichlorobenzidine including its salts is classified as carcinogenic category 2. Although 3,3′-dichlorobenzidine has been manufactured and used on an industrial scale since around 1930, and it must be assumed that the safety measures used to be inadequate, epidemiological studies do not show an increased incidence of cancer in humans. 3,3′-dichlorobenzidine seems to be an example of the fact that the carcinogenic effects of animal experiments cannot be transferred to humans.
Related links
- 2,2'-dichlorobenzidine
- 3,5-dichlorobenzidine
- 3,3'-dichlorobenzidine sulfate
- o-dianisidine (3,3′-dimethoxybenzidine)
- o-tolidine (3,3′-dimethylbenzidine)
Web links
- Main association of commercial trade associations: Procedure for determining 3,3′-dichlorobenzidine
- BAuA: Bioavailability of azo pigments after absorption via the respiratory tract
Individual evidence
- ↑ a b c d e Entry on 3,3′-dichlorobenzidine in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ Concise International Chemical Assessment Document (CICAD) for 3,3′-dichlorobenzidine , accessed November 18, 2014.
- ↑ 13th Report on Carcinogens (RoC): 3,3′-Dichlorobenzidine and Its Dihydrochloride ( Memento from November 29, 2014 in the Internet Archive ), accessed on November 18, 2014.
- ↑ Entry on 3,3′-dichlorobenzidine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values , accessed on November 2, 2015.
- ↑ Cataytic Hydrogenation of o-Nitrochlorbenzene to 3,3′-Dichlorobenzidine. In: Bulletin of the Korean Chemical Society. 23, 2002, p. 1785, doi: 10.5012 / bkcs.2002.23.12.1785 .
- ^ University of Würzburg: 3,3′-dichlorobenzidine and its salts ( Memento from June 11, 2007 in the Internet Archive ).
- ↑ Freepatentsonline: Pigment compositions in granulate form based on pigments coated with resin mixtures .
- ^ W. Herbst, K. Hunger, Industrial Organic Pigments, 3rd Edition, Wiley-VCH, Weinheim, 2004.


